Tepotinib
Tepotinib (INN/USAN), sold under the brand name Tepmetko, is a medication for the treatment of adults with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skipping.[2][3] It is a c-Met inhibitor, a type of tyrosine kinase inhibitor.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tepmetko |
| Other names | EMD-1214063 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
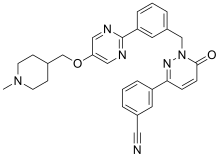
| Formula | C29H28N6O2 |
| Molar mass | 492.583 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tepotinib first received marketing approval in Japan, in March 2020, as a "line-agnostic" drug, meaning it is approved both for treatment-naive patients and for those in whom previous attempts at treatment have failed.[4] U.S. approval followed in February 2021. It is the second therapy approved by the U.S. Food and Drug Administration (FDA) to treat non-small cell lung cancer with these particular mutations, after capmatinib.
Adverse effects
The most common side effects seen in clinical trials were edema, fatigue, nausea, diarrhea, muscle aches, and shortness of breath. Like capmatinib, tepotinib can also cause interstitial lung disease and liver damage, and is toxic to a developing fetus.[2]
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214096s000lbl.pdf
- "FDA grants accelerated approval to tepotinib for metastatic non-small cell lung cancer". 2021-02-03. Retrieved 2021-02-03.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA Approves Tepmetko as the First and Only Once-daily Oral MET Inhibitor for Patients with Metastatic NSCLC with METex14 Skipping Alterations". EMD Serono (Press release). 3 February 2021. Retrieved 3 February 2021.
- "Tepmetko (Tepotinib) Approved in Japan for Advanced NSCLC with METex14 Skipping Alterations" (Press release). Merck KGaA. 2020-03-25. Retrieved 2021-02-03.
Further reading
- Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. (September 2020). "Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations". N Engl J Med. 383 (10): 931–43. doi:10.1056/NEJMoa2004407. PMID 32469185.
External links
- "Tepotinib". Drug Information Portal. U.S. National Library of Medicine.
- "Tepotinib hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
- "Tepotinib hydrochloride". NCI Drug Dictionary. National Cancer Institute.
- Clinical trial number NCT02864992 for "Tepotinib Phase II in Non-small Cell Lung Cancer (NSCLC) Harboring MET Alterations (VISION)" at ClinicalTrials.gov
