Dacarbazine
Dacarbazine (DTIC), also known as imidazole carboxamide, is a chemotherapy medication used in the treatment of melanoma and Hodgkin's lymphoma.[1] For Hodgkin's it is often used together with vinblastine, bleomycin, and doxorubicin.[1] It is given by injection into a vein.[1]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /dəˈkɑːrbəˌziːn/ |
| Trade names | DTIC-Dome, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682750 |
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | Extensive |
| Elimination half-life | 5 hours |
| Excretion | Kidney (40% as unchanged dacarbazine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.022.179 |
| Chemical and physical data | |
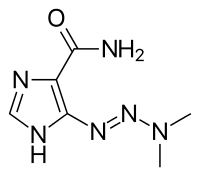
| Formula | C6H10N6O |
| Molar mass | 182.187 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects include loss of appetite, vomiting, low white blood cell count, and low platelets.[1] Other serious side effects include liver problems and allergic reactions.[1] It is unclear if use in pregnancy is safe for the baby.[1] Dacarbazine is in the alkylating agent and purine analog families of medication.[1]
Dacarbazine was approved for medical use in the United States in 1975.[1] It is on the World Health Organization's List of Essential Medicines.[2]
Medical uses
As of mid-2006, dacarbazine is commonly used as a single agent in the treatment of metastatic melanoma,[3][4] and as part of the ABVD chemotherapy regimen to treat Hodgkin's lymphoma,[5] and in the MAID regimen for sarcoma.[6][7] Dacarbazine was proven to be just as efficacious as procarbazine[8] in the German trial for paediatric Hodgkin's lymphoma, without the teratogenic effects. Thus COPDAC has replaced the former COPP regime in children for TG2 & 3 following OEPA.[9]
Side effects
Like many chemotherapy drugs, dacarbazine may have numerous serious side effects, because it interferes with normal cell growth as well as cancer cell growth. Among the most serious possible side effects are birth defects to children conceived or carried during treatment; sterility, possibly permanent; or immune suppression (reduced ability to fight infection or disease). Dacarbazine is considered to be highly emetogenic,[10] and most patients will be pre-medicated with dexamethasone and antiemetic drugs like 5-HT3 antagonist (e.g., ondansetron) and/or NK1 receptor antagonist (e.g., aprepitant). Other significant side effects include headache, fatigue and occasionally diarrhea.
The Swedish National Board of Health and Welfare has sent out a black box warning and suggests avoiding dacarbazine due to liver problems.[11]
Mechanism of action
Dacarbazine works by methylating guanine at the O-6 and N-7 positions.[12] Guanine is one of the four nucleotides that makes up DNA. The methylated DNA strands stick together such that cell division becomes impossible. This affects cancer cells more than healthy cells because cancer cells divide faster. Unfortunately however, some of the healthy cells will still be damaged.
Dacarbazine is bioactivated in liver by demethylation to "MTIC" and then to diazomethane, which is an alkylating agent.
Synthesis

History
Dacarbazine was developed by Y. Fulmer Shealy, PhD at Southern Research Institute in Birmingham, Alabama. Research was funded by a U.S. federal grant. Dacarbazine gained FDA approval in May 1975 as DTIC-Dome. The drug was initially marketed by Bayer.
Suppliers
Bayer continues to supply DTIC-Dome. There are also generic versions of dacarbazine available from APP, Bedford, Mayne Pharma (now Hospira) and Teva.
See also
References
- "Dacarbazine". The American Society of Health-System Pharmacists. Archived from the original on 11 September 2017. Retrieved 8 December 2016.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Serrone, L; Zeuli, M; Sega, FM; Cognetti, F (March 2000). "Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview". Journal of Experimental & Clinical Cancer Research : CR. 19 (1): 21–34. PMID 10840932.
- Bhatia, S; Tykodi, SS; Thompson, JA (May 2009). "Treatment of metastatic melanoma: an overview". Oncology (Williston Park, N.Y.). 23 (6): 488–96. PMC 2737459. PMID 19544689.
- Rueda Domínguez, A; Márquez, A; Gumá, J; Llanos, M; Herrero, J; de Las Nieves, MA; Miramón, J; Alba, E (December 2004). "Treatment of stage I and II Hodgkin's lymphoma with ABVD chemotherapy: results after 7 years of a prospective study". Annals of Oncology. 15 (12): 1798–804. doi:10.1093/annonc/mdh465. PMID 15550585.
- Elias, A; Ryan, L; Aisner, J; Antman, KH (April 1990). "Mesna, doxorubicin, ifosfamide, dacarbazine (MAID) regimen for adults with advanced sarcoma". Seminars in Oncology. 17 (2 Suppl 4): 41–9. PMID 2110385.
- Pearl, ML; Inagami, M; McCauley, DL; Valea, FA; Chalas, E; Fischer, M (2001). "Mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) chemotherapy for gynecological sarcomas". International Journal of Gynecological Cancer. 12 (6): 745–8. doi:10.1046/j.1525-1438.2002.01139.x. PMID 12445253. S2CID 25246937.
- Jelić, S; Babovic, N; Kovcin, V; Milicevic, N; Milanovic, N; Popov, I; Radosavljevic, D (February 2002). "Comparison of the efficacy of two different dosage dacarbazine-based regimens and two regimens without dacarbazine in metastatic melanoma: a single-centre randomized four-arm study". Melanoma Research. 12 (1): 91–8. doi:10.1097/00008390-200202000-00013. PMID 11828263. S2CID 32031568.
- Mauz-Körholz, C; Hasenclever, D; Dörffel, W; Ruschke, K; Pelz, T; Voigt, A; Stiefel, M; Winkler, M; Vilser, C; Dieckmann, K; Karlén, J; Bergsträsser, E; Fosså, A; Mann, G; Hummel, M; Klapper, W; Stein, H; Vordermark, D; Kluge, R; Körholz, D (10 August 2010). "Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: the GPOH-HD-2002 study". Journal of Clinical Oncology. 28 (23): 3680–6. doi:10.1200/jco.2009.26.9381. PMID 20625128.
- Aoki, S; Iihara, H; Nishigaki, M; Imanishi, Y; Yamauchi, K; Ishihara, M; Kitaichi, K; Itoh, Y (January 2013). "Difference in the emetic control among highly emetogenic chemotherapy regimens: Implementation for appropriate use of aprepitant". Molecular and Clinical Oncology. 1 (1): 41–46. doi:10.3892/mco.2012.15. PMC 3956247. PMID 24649120.
- "Archived copy". Archived from the original on October 1, 2011. Retrieved August 19, 2011.CS1 maint: archived copy as title (link)
- Kewitz, S; Stiefel, M; Kramm, CM; Staege, MS (January 2014). "Impact of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation and MGMT expression on dacarbazine resistance of Hodgkin's lymphoma cells". Leukemia Research. 38 (1): 138–43. doi:10.1016/j.leukres.2013.11.001. PMID 24284332.
External links
- "Dacarbazine". Drug Information Portal. U.S. National Library of Medicine.