Influenza A virus subtype H9N2
Influenza A virus subtype H9N2 (A/H9N2) is a subtype of the species Influenza A virus (bird flu virus).[1][2]
| Influenza A virus subtype H9N2 | |
|---|---|
 | |
| Influenza A - late passage | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Insthoviricetes |
| Order: | Articulavirales |
| Family: | Orthomyxoviridae |
| Genus: | Alphainfluenzavirus |
| Species: | Influenza A virus |
| Serotype: | Influenza A virus subtype H9N2 |
Over the years the H9N2 influenza strain caused illness in several children aged nine months to 5 years in Hong Kong with the latest occurring in December 2020.[3]
Infection in birds
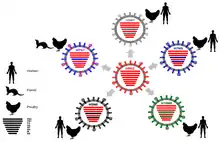
H9N2 is the most common subtype of influenza viruses in Chinese chickens and thus causes great economic loss for the poultry industry, even under the long-term vaccination programs. Recent human infections with avian influenza virus revealed that H9N2 is the gene donor for H7N9 and H10N8 viruses that are infecting humans too. The crucial role of H9N2 viruses due to the wide host range, adaptation to both poultry and mammals and extensive gene reassortment. In China, which is regarded as a breeding ground of avian influenza viruses, the H9N2 virus has been detected in multiple avian species, including chicken, duck, quail, pheasant, partridge, pigeon, silky chicken, chukar and egret.[4]
Epidemiological and genetic studies revealed that the hemagglutinin (HA) gene of the H9N2 influenza viruses could be divided into Eurasian avian and American avian lineages. The Eurasian avian lineage involved three distinct lineages, including A/chicken/Beijing/1/94-like (BJ/94-like), A/quail/Hong Kong/G1/97-like (G1-like), and A/duck/Hong Kong/Y439/97 (Y439-like).[4]
Transmission from chicken to human
The H9N2 influenza virus can be transmitted by air droplet, dust, feed, or water. Chickens usually seemed to be healthy after the infection but some of them do show depression and ruffled feathers. The virus replicates itself in the trachea. It makes chickens more susceptible to secondary infections, especially Escherichia coli infections with a mortality rate of at least 10%. Also, the trachea or bronchi are easily embolized by mucus when the ventilation is poor, leading to severe respiratory disease and death.[4]
Antigenicity
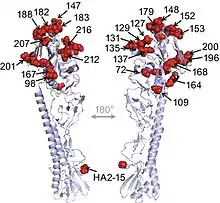
H9N2 viruses isolated from chickens in China showed antigenic drift that evolved into distinct antigenic groups. This antigenic drift may have led to immunization failure and may explain the current prevalence of the H9N2 influenza virus in China. The identification of amino acids in H9 antigenic sites revealed different distribution of antigenic areas among other subtypes. Multiple amino acid positions in HA protein related to the antigenicity of H9N2 viruses were identified, most of which located in the distal head of the HA trimer. H9N2 influenza virus has been recognized to reassort with multiple other subtypes, including H6N1, H6N2, and H5N1 viruses. Moreover, H7N9 influenza viruses continued to reassort with circulating H9N2 viruses, resulting in multiple genotypes of H7N9 viruses. The contribution of H9N2 genes, especially ribonucleoprotein (RNP) genes, to the infection in human needs to be determined.[4]
Sources
- Guan Y, Shortridge KF, Krauss S, Webster RG (August 1999). "Molecular characterization of H9N2 influenza viruses: were they the donors of the "internal" genes of H5N1 viruses in Hong Kong?". Proc. Natl. Acad. Sci. U.S.A. 96 (16): 9363–7. Bibcode:1999PNAS...96.9363G. doi:10.1073/pnas.96.16.9363. PMC 17788. PMID 10430948.
- NAID NIH Archived 2010-03-06 at the Wayback Machine
- "COMMUNICABLE DISEASE THREATS REPORT Week 3, 17-23 January 2021" (PDF). europa. 23 Jan 2021. Retrieved 27 Jan 2021.
- Sun Y, Liu J (2015). "H9N2 influenza virus in China: a cause of concern". Protein Cell. 6 (1): 18–25. doi:10.1007/s13238-014-0111-7. PMC 4286136. PMID 25384439.
Further reading
- Butt AM, Siddique S, Idrees M, Tong Y (November 2010). "Avian influenza A (H9N2): computational molecular analysis and phylogenetic characterization of viral surface proteins isolated between 1997 and 2009 from the human population". Virology Journal. 7 (1): 319. doi:10.1186/1743-422X-7-319. PMC 2994543. PMID 21078137.
Our findings support the continuous evolution of avian H9N2 viruses towards human as host and are in favor of effective surveillance and better characterization studies to address the issue.
- Li C, Yu K, Tian G, et al. (September 2005). "Evolution of H9N2 influenza viruses from domestic poultry in Mainland China". Virology. 340 (1): 70–83. doi:10.1016/j.virol.2005.06.025. PMID 16026813.
Our findings suggest that urgent attention should be paid to the control of H9N2 influenza viruses in animals and to the human's influenza pandemic preparedness.
- Centers for Disease Control and Prevention (CDC) - Influenza A(H9N2) infections in Hong Kong published April 8, 1999.
- Uyeki TM, Chong YH, Katz JM, et al. (February 2002). "Lack of evidence for human-to-human transmission of avian influenza A (H9N2) viruses in Hong Kong, China 1999". Emerging Infect. Dis. 8 (2): 154–9. doi:10.3201/eid0802.010148. PMC 2732440. PMID 11897066.
- People's Daily Online - Hong Kong reports human case of H9N2 published March 20, 2007.
- Xinhua News Agency - HK girl infected with rare but mild A/H9N2 flu virus published December 23, 2009.
External links
- Influenza Research Database Database of influenza genomic sequences and related information.