Magnesium fluoride
Magnesium fluoride is an inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.
 | |
| Names | |
|---|---|
| Other names
Sellaite Irtran-1 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.086 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| MgF2 | |
| Molar mass | 62.3018 g/mol |
| Appearance | White tetragonal crystals |
| Density | 3.148 g/cm3 |
| Melting point | 1,263 °C (2,305 °F; 1,536 K) |
| Boiling point | 2,260 °C (4,100 °F; 2,530 K) |
| 0.013 g/100 mL | |
Solubility product (Ksp) |
5.16⋅10−11 |
| Solubility | Insoluble in ethanol |
| −22.7⋅10−6 cm3/mol | |
Refractive index (nD) |
1.37397 |
| Structure | |
| Rutile (tetragonal), tP6 | |
| P42/mnm, No. 136 | |
| Thermochemistry | |
Heat capacity (C) |
61.6 J⋅mol−1⋅K−1 |
Std molar entropy (S |
57.2 J⋅mol−1⋅K−1 |
Std enthalpy of formation (ΔfH⦵298) |
−1124.2 kJ⋅mol−1 |
Gibbs free energy (ΔfG˚) |
−1071 kJ/mol |
| Hazards[2][3] | |
| Safety data sheet | ChemicalBook |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H303, H315, H319, H335 | |
| P261, P304+340, P305+351+338, P405 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2330 (rat, oral) |
| Related compounds | |
Other anions |
Magnesium chloride Magnesium bromide Magnesium iodide |
Other cations |
Beryllium fluoride Calcium fluoride Strontium fluoride Barium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production and structure
Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride:
- MgO + (NH4)HF2 → MgF2 + NH3 + H2O
Related metathesis reactions are also feasible.
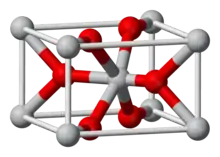
The compound crystallizes as tetragonal birefringent crystals. The structure of the compound is similar to that in rutile, featuring octahedral Mg2+ centers and 3-coordinate fluoride centres.[4]
Uses
Optics
Magnesium fluoride is transparent over an extremely wide range of wavelengths. Windows, lenses, and prisms made of this material can be used over the entire range of wavelengths from 0.120 μm (vacuum ultraviolet) to 8.0 μm (infrared). High-quality, synthetic magnesium fluoride is one of two materials (the other being lithium fluoride) that will transmit in the vacuum ultraviolet range at 121 nm (Lyman alpha). Lower-grade magnesium fluoride is inferior to calcium fluoride in the infrared range.
Magnesium fluoride is tough and polishes well but is slightly birefringent and should therefore be cut with the optic axis perpendicular to the plane of the window or lens.[4] Due to its suitable refractive index of 1.37, magnesium fluoride is commonly applied in thin layers to the surfaces of optical elements as an inexpensive anti-reflective coating. Its Verdet constant is 0.00810 arcmin⋅G–1⋅cm–1 at 632.8 nm.[5]
Safety
Chronic exposure to magnesium fluoride may affect the skeleton, kidneys, central nervous system, respiratory system, eyes and skin, and may cause or aggravate attacks of asthma.[6]
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–67, 1363, ISBN 0-8493-0594-2
- "Magnesium Fluoride Material Safety Data Sheet". Science Labs. May 21, 2013. Retrieved October 13, 2017.
- "Magnesium fluoride". CAS DataBase List. ChemicalBook. Retrieved October 13, 2017.
- Aigueperse, Jean; Mollard, Paul; Devilliers, Didier; Chemla, Marius; Faron, Robert; Romano, René; Cuer, Jean Pierre (2000). "Fluorine Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_307.
- J. Chem. Soc., Faraday Trans., 1996, 92, 2753 - 2757. doi:10.1039/FT9969202753
- "Magnesium Fluoride Material Safety Data Sheet". ESPI Metals. August 2004. Archived from the original on 2017-10-28. Retrieved October 13, 2017.
External links
- A java applet showing the effect of MgF2 on a lens
- Infrared windows at Lawrence Berkeley National Laboratory
- National Pollutant Inventory - Fluoride and compounds fact sheet
- Crystran Data Crystran MSDS
