Xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas.[3] It is produced by the chemical reaction of xenon with fluorine, F
2, according to the chemical equation:[4][5]
- Xe + 2 F
2 → XeF
4
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Xenon tetrafluoride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.858 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| XeF 4 | |||
| Molar mass | 207.2836 g mol−1 | ||
| Appearance | White solid | ||
| Density | 4.040 g cm−3, solid | ||
| Melting point | 117 °C (243 °F; 390 K) sublimes[1] | ||
| Reacts | |||
| Structure | |||
| D4h | |||
| square planar | |||
| 0 D | |||
| Thermochemistry | |||
Std molar entropy (S |
146 J·mol−1·K−1[2] | ||
Std enthalpy of formation (ΔfH⦵298) |
−251 kJ·mol−1[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
This reaction is exothermic, releasing an energy of 251 kJ/mol.[3]
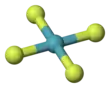
Xenon tetrafluoride is a colorless crystalline substance. Its structure was determined by both NMR spectroscopy and X-ray crystallography in 1963.[6][7] The structure is square planar, as has been confirmed by neutron diffraction studies,[8] According to VSEPR theory, in addition to four fluoride ligands, the xenon center has two lone pairs of electrons. These lone pairs are mutually trans.
Synthesis
Xenon tetrafluoride is produced by heating a mixture of xenon and fluorine in a 1:5 ratio in a nickel container to 400 °C. Some xenon hexafluoride, XeF
6, is also produced, and this production is increased with an increased fluorine concentration in the input mixture.[9] The nickel is not a catalyst for this reaction; nickel containers are used because they react with fluorine to form a protective, non-peeling layer of nickel(II) fluoride NiF
2 on their interior surfaces.
Reactions
Xenon tetrafluoride hydrolyzes at low temperatures to form elemental xenon, oxygen, hydrofluoric acid, and aqueous xenon trioxide.[10]
Reaction with tetramethylammonium fluoride gives tetramethylammonium pentafluoroxenate, which contains the pentagonal XeF−
5 anion. The XeF−
5 anion is also formed by reaction with caesium fluoride:[11]
- CsF + XeF
4 → CsXeF
5
Reaction with bismuth pentafluoride (BiF
5) forms the XeF+
3 cation:[12]
- BiF
5 + XeF
4 → XeF3BiF6
The XeF+
3 cation in the salt XeF3Sb2F11 has been characterized by NMR spectroscopy.[13]
At 400 °C, XeF
4 reacts with xenon to form XeF
2:[9]
- XeF4 + Xe → 2 XeF2
The reaction of xenon tetrafluoride with platinum yields platinum tetrafluoride and xenon:[9]
- XeF4 + Pt → PtF4 + Xe
Applications
Xenon tetrafluoride has few applications. It has been shown to degrade silicone rubber for analyzing trace metal impurities in the rubber. XeF
4 reacts with the silicone to form simple gaseous products, leaving a residue of metal impurities.[14]
References
- Holleman, Arnold F.; Wiberg, Egon (2001). Wiberg, Nils (ed.). Inorganic Chemistry. Translated by Eagleson, Mary; Brewer, William. Academic Press. p. 394. ISBN 0-12-352651-5.
- Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- Zumdahl (2007). Chemistry. Boston: Houghton Mifflin. p. 243. ISBN 0-618-52844-X.
- Claassen, H. H.; Selig, H.; Malm, J. G. (1962). "Xenon Tetrafluoride". J. Am. Chem. Soc. 84 (18): 3593. doi:10.1021/ja00877a042.
- Chernick, C. L.; Claassen, H. H.; Fields, P. R.; Hyman, H. H.; Malm, J. G.; Manning, W. M.; Matheson, M. S.; Quarterman, L. A.; Schreiner, F.; Selig, H. H.; Sheft, I.; Siegel, S.; Sloth, E. N.; Stein, L.; Studier, M. H.; Weeks, J. L.; Zirin, M. H. (1962). "Fluorine Compounds of Xenon and Radon". Science. 138 (3537): 136–138. Bibcode:1962Sci...138..136C. doi:10.1126/science.138.3537.136. PMID 17818399.
- Brown, Thomas H.; Whipple, E. B.; Verdier, Peter H. (1963). "Xenon Tetrafluoride: Fluorine-19 High-Resolution Magnetic Resonance Spectrum". Science. 140 (3563): 178. Bibcode:1963Sci...140..178B. doi:10.1126/science.140.3563.178. PMID 17819836.
- Ibers, James A.; Hamilton, Walter C. (1963). "Xenon Tetrafluoride: Crystal Structure". Science. 139 (3550): 106–107. Bibcode:1963Sci...139..106I. doi:10.1126/science.139.3550.106. PMID 17798707.
- Burns, John H.; Agron, P. A.; Levy, Henri A (1963). "Xenon Tetrafluoride Molecule and Its Thermal Motion: A Neutron Diffraction Study". Science. 139 (3560): 1208–1209. Bibcode:1963Sci...139.1208B. doi:10.1126/science.139.3560.1208. PMID 17757912.
- Bard, Allen J.; Parsons, Roger; Jordan, Joseph; International Union of Pure and Applied Chemistry (1985). Standard Potentials in Aqueous Solution. CRC Press. pp. 767–768. ISBN 0-8247-7291-1.
- Williamson; Koch, C. W. (Mar 1963). "Xenon Tetrafluoride: Reaction with Aqueous Solutions". Science. 139 (3559): 1046–1047. Bibcode:1963Sci...139.1046W. doi:10.1126/science.139.3559.1046. ISSN 0036-8075. PMID 17812981.
- Harding, Charlie; Johnson, David Arthur; Janes, Rob (2002). Elements of the p Block. Molecular World. 9. Royal Society of Chemistry. p. 93. ISBN 0-85404-690-9.
- Suzuki, Hitomi; Matano, Yoshihiro (2001). Organobismuth chemistry. Elsevier. p. 8. ISBN 0-444-20528-4.
- Gillespie, R. J.; Landa, B.; Schrobilgen, G. J. (1971). "Trifluoroxenon(IV) µ-fluoro-bispentafluoroantimonate(V): the XeF+
3 cation". Journal of the Chemical Society D: Chemical Communications (23): 1543–1544. doi:10.1039/C29710001543. - Rigin, V.; Skvortsov, N. K.; Rigin, V. V. (March 1997). "Xenon tetrafluoride as a decomposition agent for silicone rubber for isolation and atomic emission spectrometric determination of trace metals". Analytica Chimica Acta. 340 (1–3): 1–3. doi:10.1016/S0003-2670(96)00563-6.