Mercury(I) fluoride
Mercury(I) fluoride or mercurous fluoride is the chemical compound composed of mercury and fluorine with the formula Hg2F2. It consists of small yellow cubic crystals, which turn black when exposed to light.[1]
-fluoride-3D-vdW.png.webp) | |
| Names | |
|---|---|
| IUPAC name
Dimercury difluoride | |
| Other names
Mercury(I) fluoride Mercurous fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.034.302 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| Hg2F2 | |
| Molar mass | 439.177 g/mol |
| Appearance | yellow cubic crystals |
| Density | 8.73 g/cm³, solid |
| decomposes[1] | |
| −26.5·10−6 cm3/mol | |
| Hazards | |
EU classification (DSD) (outdated) |
Very toxic (T+) Dangerous for the environment (N) |
| R-phrases (outdated) | R26/27/28, R33, R50/53 |
| S-phrases (outdated) | S13, S28, S45, S60, S61[2] |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Other anions |
Mercury(I) chloride Mercury(I) bromide Mercury(I) iodide |
Other cations |
Zinc fluoride Cadmium fluoride |
Related compounds |
Mercury(II) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Mercury(I) fluoride is prepared by the reaction of mercury(I) carbonate with hydrofluoric acid:
- Hg2CO3 + 2 HF → Hg2F2 + CO2 + H2O
Reactions
When added to water, mercury(I) fluoride hydrolyzes to elemental liquid mercury, mercury(II) oxide, and hydrofluoric acid:[1]
- Hg2F2 + H2O → Hg + HgO + 2 HF
It can be used in the Swarts reaction to convert alkyl halides into alkyl fluorides:[3]
Structure
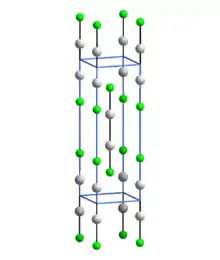
In common with other Hg(I) (mercurous) compounds which contain linear X-Hg-Hg-X units, Hg2F2 contains linear FHg2F units with an Hg-Hg bond length of 251 pm (Hg-Hg in the metal is 300 pm) and an Hg-F bond length of 214 pm.[4] The overall coordination of each Hg atom is a distorted octahedron; in addition to the bonded F and other Hg of the molecule, there are four other F atoms at 272 pm.[4] The compound is often formulated as Hg22+ 2F−.[5]
References
- Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 256, ISBN 0-8493-8671-3, retrieved 2008-06-17
- 339318 Mercury(I) fluoride technical grade, Sigma-Aldrich, retrieved 2008-06-17
- Beyer, Hans; Walter, Wolfgang; Lloyd, Douglas (1997), Organic Chemistry, Horwood Publishing, p. 136, ISBN 1-898563-37-3, retrieved 2008-06-17
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5

