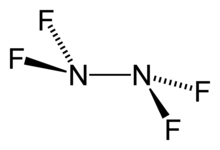
Tetrafluorohydrazine
Tetrafluorohydrazine or dinitrogen tetrafluoride, N2F4, is a colourless, reactive inorganic gas. It is a fluorinated analog of hydrazine. It is a highly hazardous chemical that explodes in the presence of organic materials.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1,2,2-tetrafluorohydrazine | |
| Other names
dinitrogen tetrafluoride, perfluorohydrazine, UN 1955 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.091 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N2F4 | |
| Molar mass | 104.01 g mol−1 |
| Melting point | −164.5 °C (−264.1 °F; 108.6 K) [1] |
| Boiling point | −73 °C (−99 °F; 200 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrafluorohydrazine is manufactured from nitrogen trifluoride using an iron catalyst or iron(II) fluoride. It is used in some chemical syntheses, as a precursor or a catalyst.
Tetrafluorohydrazine was considered for use as a high-energy liquid oxidizer in some never-flown rocket fuel formulas in 1959.[2]
Properties
Tetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride.[3]
- N2F4 ⇌ 2 NF2•
At room temperature N2F4 is mostly associated with only 0.7% in the form of NF2 at 5mm Hg pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of NF2.[4]
The energy needed to break the N-N bond in N2F4 is 20.8 kcal/mol, with an entropy change of 38.6 eu.[4] For comparison, the dissociation energy of the N-N bond is 14.6 kcal/mol in N2O4, 10.2 kcal/mol in N2O2, and 60 kcal/mol in N2H4. The enthalpy of formation of N2F4 (ΔHf) is 34.421 kJ/mol.[5]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Tetrafluorohydrazine at DTIC.mil archived March 12, 2007
- Jäger, Susanne; von Jouanne, Jörn; Keller-Rudek, Hannelore; Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Rupecht, Sigrid; Vanecek, Hans; Wagner, Joachim (1986). Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Ruprecht, Sigrid; Wagner, Joachim (eds.). F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry. 4. Berlin: Springer. p. 162. doi:10.1007/978-3-662-06339-2. ISBN 978-3-662-06341-5. Retrieved 29 August 2015.
- Bohn, Robert K.; Bauer, Simon Harvey (February 1967). "An electron diffraction study of the structures of NF2 and N2F4". Inorganic Chemistry. 6 (2): 304–309. doi:10.1021/ic50048a024. molecule dimensions and angles
- "Nitrogen difluoride NF2(g)". www.chem.msu.su.