Organobismuth chemistry
Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. Applications are few.[1] The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi.[2] The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Challenger in 1934 (using Ph3Bi(OH)2).[3] Knowledge about methylated species of bismuth in environmental and biological media is limited.[4]
OrganoBi(III) compounds
Structure
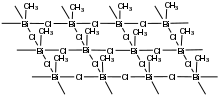
Triorganobismuth(III) compounds are monomeric with pyramidal structures reminiscent of organophosphorus(III) chemistry. The halides however adopt hypervalent structures. This trend is illustrated by the sheet-like structure adopted by methylbismuth dichloride.[5]
Organobismuth heterocycles are based on Bi(III). The cyclic compound bismole, a structural analog of pyrrole, has not been isolated, but substituted bismoles are known.[6] Bismabenzene has been detected in the laboratory.[7]
Synthesis
Organobismuth(III) compounds are often prepared from BiCl3 by substitution with the corresponding carbon nucleophiles, generally of the form of a Grignard reagent or alkyl/aryl lithium species:
- BiCl3 + 3 RMgX → R3Bi + 3 MgXCl
Triaryl bismuth(III) compounds are typically air-stable crystalline solids. The substituents on the triarylbismuth center can be modified.[8]
_Reagents.png.webp)
Reactions
Triarylbismuth compounds have very limited use in organic synthesis.[9] They react with acylchlorides under Pd(0) catalysis to form a variety of phenyl ketones.[10] Tricyclopropylbismuth(III) reagents react with aryl halides and triflates under Pd(0) catalysis in a similar fashion to afford a variety of aryl and heteroaryl cyclopropanes.[11]
.png.webp)
Triphenylbismuth undergoes redistribution with its trihalide to give the mixed derivatives such as diphenylbismuth chloride (Ph2BiCl).[12] Such reactions proceed more readily than for the lighter congeners.
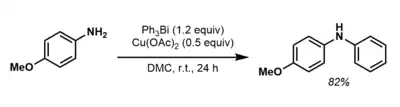
Triaryl bismuth(III) compounds may also be employed in C-N bond forming transformations with an appropriate metal co-catalyst. For instance, Barton and coworkers demonstrated that amines could be N-arylated with a bismuth(III) reagent in the presence of copper(II) salt.[13]
OrganoBi(V) compounds
Structure
Organobismuth(V) compounds of the type Ar5Bi adopt square pyramidal structures. The pentaphenyl compound is deeply colored and thermochromic, possibly because of an equilibrium between square pyramidal and trigonal bipyramidal structures.[14]
Synthesis
Organobismuth(V) complexes may be accessed directly from organobismuth(III) through oxidative addition to a halogen then displacement of the newly formed bismuth-halogen bond for a bismuth-carbon bond with an alkyl or aryl lithium or Grignard reagent.
Bi(V) compounds can be accessed through Bi(III) compounds for example:
- Me3Bi + SO2Cl2 → Me3BiCl2 + SO2
- Me3BiCl2 + 2 MeLi → Me5Bi + 2 LiCl
Bi(V) easily forms an onium ion for example by protonation with p-toluenesulfonic acid:[15]
- Ph5Bi + HO3SAr → Ph4Bi+[O3SAr−]
Pentaphenylbismuth forms an ate complex upon treatment with phenyl lithium:[16]
- Ph5Bi + PhLi → Li+[Ph6Bi−]
The thermal stability of R5M compounds decrease in the order As > Sb > Bi. The aryl compounds are more stable than alkyl compounds. Me5Bi decomposes explosively at 20 °C.
Reactions
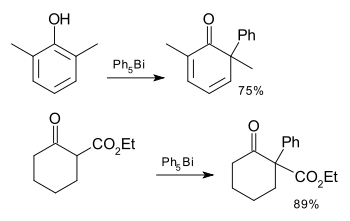
Compared to the lighter congeners, Bi(V) compounds are oxidizing. The compounds Ph3Bi(OOtBu)2, Ph3BiCO3 and (Ph3BiCl)2O have been investigated for the oxidation of oximes, thiols, phenols, and phosphines.[17] Compounds such as Ph5Bi and Ph3BiCl2 have been used in the arylation of arene compounds and 1,3-dicarbonyl compounds:[18]
The above transformation proceeds through in an asynchronous concerted fashion from the O-bound organobismuth(V) reagent after loss of an aryl group. A triarylbismuth(III) complex forms concomitantly.[19] Regioselectivity of this transformation is guided by the directing ability of adjacent lewis basic functionalities. It is important to note that in the above arylation, a full equivalent of the pentavalent bismuth compound is required for the arylation reaction therefore leaving four ligands on bismuth inactive for further arylations. Catalytic manifolds of this chemistry are challenging due in part to the reoxidation of Bi(III) to Bi(V). For more examples of bismuth mediated arylations, see the cited review.[20]

References
- "Bismuth-Mediated Organic Reactions", Ollevier, T. Ed., Topics in Current Chemistry, 2012, Springer Verlag, Berlin, vol. 311, 277 p. https://www.springer.com/chemistry/organic+chemistry/book/978-3-642-27238-7, Bismuth Reagents and Catalysts in Organic Synthesis Axel Jacobi von Wangelin in Transition Metals for Organic Synthesis Building Blocks and Fine Chemicals Beller, Matthias / Bolm, Carsten (eds.)
- C. Elschenbroich, A. Salzer Organometallics : A Concise Introduction (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- Organo-derivatives of bismuth and thallium Frederick Challenger and Oswald V. Richards, J. Chem. Soc., 1934, 405 doi:10.1039/JR9340000405
- Filella, M. (2010-03-15). "Alkyl derivatives of bismuth in the environmental and biological media". Metal Ions in Life Sciences. Cambridge: RSC publishing. 7: 303–317. doi:10.1039/9781849730822-00303. ISBN 978-1-84755-177-1. PMID 20877811.
- Althaus, Henrik; Breunig, Hans Joachim; Lork, Enno (2001). "Syntheses and Chemistry of Methylantimony and Methylbismuth Dihalides: An Extended Two-Dimensional Framework in the Crystal Structure of CH3BiCl2 and Molecular Units in the Structures of [CH3ECl2(2,2'-bipyridine)] (E = Sb, Bi)". Organometallics. 20 (3): 586–589. doi:10.1021/om000749i.
- Caster, Kenneth C. "Arsoles, stiboles, and bismoles" Clive W. Bird, ed. Comprehensive Heterocyclic Chemistry II (1996), 2, 857-902.
- Gagnon, Alexandre (2017). "Organobismuth Reagents: Synthesis, Properties and Applications in Organic Synthesis". Synthesis. 49 (8): 1707–1745. doi:10.1055/s-0036-1589482.
- Hébert, Martin; Petiot, Pauline; Benoit, Emeline; Dansereau, Julien; Ahmad, Tabinda; Le Roch, Adrien; Ottenwaelder, Xavier; Gagnon, Alexandre (2016). "Synthesis of Highly Functionalized Triarylbismuthines by Functional Group Manipulation and Use in Palladium- and Copper-Catalyzed Arylation Reactions". The Journal of Organic Chemistry. 81 (13): 5401–5416. doi:10.1021/acs.joc.6b00767. PMID 27231755.
- Finet, Jean Pierre (1989). "Arylation Reactions with Organobismuth Reagents". Chemical Reviews. 89 (7): 1487–1501. doi:10.1021/cr00097a005.
- "ScienceDirect". www.sciencedirect.com. Retrieved 2019-05-08.
- Gagnon, Alexandre; Duplessis, Martin; Alsabeh, Pamela; Barabé, Francis (2008). "Palladium-Catalyzed Cross-Coupling Reaction of Tricyclopropylbismuth with Aryl Halides and Triflates". The Journal of Organic Chemistry. 73 (9): 3604–3607. doi:10.1021/jo702377h. PMID 18363369.
- Barton, Derek H.R.; Bhatnagar, Neerja Yadav; Finet, Jean-Pierre; Motherwell, William B. (1986). "Pentavalent Organobismuth Reagents. Part vi. Comparative Migratory Aptitudes of Aryl Groups in the Arylation of Phenols and Enols by Pentavalent Bismuth Reagents". Tetrahedron. 42 (12): 3111–3122. doi:10.1016/S0040-4020(01)87378-6.
- Barton, Derek H. R. (1987). "Copper Salts Catalysis of N-Phenylation of Amines by Trivalent Organobismuth Compounds". Tetrahedron Letters. 28 (8): 887–890. doi:10.1016/S0040-4039(01)81015-7.
- Schmuck, Arno; Seppelt, Konrad (1989). "Strukturen von Pentaarylbismut-Verbindungen". Chemische Berichte. 122 (5): 803–808. doi:10.1002/cber.19891220502.
- Barton, Derek H. R.; Charpiot, Brigitte; Dau, Elise Tran Huu; Motherwell, William B.; Pascard, Claudine; Pichon, Clotilde (1984). "Structural Studies of Crystalline Pentavalent Organobismuth Compounds". Helvetica Chimica Acta. 67 (2): 586–599. doi:10.1002/hlca.19840670227.
- Wallenhauer, Stephan; Leopold, Dieter; Seppelt, Konrad (1993). "Hexacoordinate Organobismuth Compounds". Inorganic Chemistry. 32 (18): 3948–3951. doi:10.1021/ic00070a029.
- Organobismuth Chemistry Hitomi Suzuki, Yoshihiro Matano Elsevier, 2001
- Bismuth(V) reagents in organic synthesis Derek H.R. Barton and Jean-Pierre Finet Pure Appl. Chem., Vol. 59, No. 8, pp. 937—946, 1987.
- "ScienceDirect". www.sciencedirect.com. Retrieved 2019-05-08.
- Konopelski, Joseph P. (2001). "Arylation with organolead and organobismuth reagents". Tetrahedron. 57 (27): 5683–5705. doi:10.1016/S0040-4020(01)00385-4.