Organovanadium chemistry
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon to vanadium (V) chemical bond.[1] Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.[2]
Oxidation states for vanadium are +2, +3, +4 and +5. Low valency vanadium is usually stabilized with carbonyl ligands. Oxo derivatives are relatively common, unlike the organic complexes of neighboring elements.
Compound classes
Carbonyls
Vanadium carbonyl can be prepared by reductive carbonylation of vanadium salts:
- 4 Na + VCl3 + 6 CO → Na[V(CO)6] + 3 NaCl
The salt can be oxidized to the 17e binary carbonyl V(CO)6.
Cyclopentadienyl derivatives
Vanadocene dichloride, the first organovanadium complexes to be reported,[3] is prepared from sodium cyclopentadienyl and vanadium tetrachloride:
- 2 NaC5H5 + VCl4 → VCp2Cl2 + 2NaCl
Reduction of this compound gives the parent vanadocene (Cp)2V:
- VCp2Cl2 + LiAlH4 → V(Cp)2
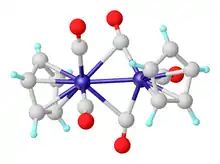
Vanadocene is the lightest transition metal metallocene that is isolable at room temperature.[5] Vanadocene reacts with high pressures of carbon monoxide to give CpV(CO)4.[6] Photolysis of the tetracarbonyl gives Cp2V2(CO)5. Several analogous indenyl complexes are known.
Monocyclopentadienyl vanadium chlorides include CpVCl3 and the diamagnetic CpVOCl2.
Arene complexes
Vanadium forms a variety of arene complexes, e.g. with benzene:
- VCl4 + AlCl3 + C6H6 → [V(η6C6H6)2][AlH4]
- [V(η6C6H6)2][AlH4] + H2O → V(η6C6H6)2
Alkyl and aryl derivatives
A handful of alkyl and aryl complexes exist for example with mesitylene groups:
- VCl3(THF)3 + (mes)MgBr → V(mes)3(THF)
- V(mes)3(THF) + LiMes → Li[V(mes)4]
- Li[V(mes)4] + air → V(mes)4(THF)
The tetrakis(norbornyl) complex is also known.
Vanadium oxytrichloride is a starting material for vanadium(V) compounds:
- VOCl3 + Li(mes) → Li[VO(mes)3]
- Li[VO(mes)3] + chloranil → VO(mes)3
- VOCl3 + ZnPh2 → VOPhCl2
Catalysts and reagents
Well-defined vanadium compounds do not appear as catalysts in any commercial process.[7] However organovanadium species are clearly implicated as catalysts for the production of butadiene-based rubbers. These catalysts are generated in situ by treating soluble coordination complexes such as vanadium(III) acetylacetonate with organoaluminium activators.[8] [9]
References
- Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. 1997
- Kotohiro Nomura, Shu Zhang (2011). "Design of Vanadium Complex Catalysts for Precise Olefin Polymerization". Chem. Rev. 111: 2342–2362. doi:10.1021/cr100207h.CS1 maint: uses authors parameter (link)
- Wilkinson, G.; Birmingham, J. G. (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". J. Am. Chem. Soc. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- Huffman, J. C.; Lewis, L. N.; Caulton, K. G. (1980). "A Donor Semibridge? Molecular Structures of Dicyclopentadienyldivanadiumtetracarbonyltriphenylphosphine and Dicyclopentadienyldivanadiumpentacarbonyl". Inorganic Chemistry. 19: 2755–2762. doi:10.1021/ic50211a052.CS1 maint: uses authors parameter (link)
- Robert Choukroun, Christian Lorber (2005). "Adventures in Vanadocene Chemistry". Eur. J. Inorg. Chem.: 4683–4692. doi:10.1002/ejic.200500371.
- King, R.B.; Stone, F.G.A (1963). "Cyclopentadienyl Metal Carbonyls and Some Derivatives". Inorg. Synth. 7: 99. doi:10.1002/9780470132388.ch31.
- Toshikazu Hirao (1997). "Vanadium in Modern Organic Synthesis". Chemical Reviews. 97: 2707. doi:10.1021/cr960014g.
- Kotohiro Nomura, Shu Zhang (2011). "Design of Vanadium Complex Catalysts for Precise Olefin Polymerization". Chem. Rev. 111: 2342–2362. doi:10.1021/cr100207h.CS1 maint: uses authors parameter (link)
- Werner Obrecht, Jean-Pierre Lambert, Michael Happ, Christiane Oppenheimer-Stix, John Dunn, Ralf Krüger (2012). "Rubber, 4. Emulsion Rubber". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o23_o01.CS1 maint: uses authors parameter (link)