Orthacanthus
Orthacanthus is an extinct genus of fresh-water xenacanthid sharks, named by Louis Agassiz in 1836,[2] ranging from the Upper Carboniferous until the Lower Permian.[1] Orthacanthus lived in marine environments and had a nektobenthic life habitat, with a carnivorous diet.[3] Multiple sources have also discovered evidence of cannibalism in the diet of Orthacanthus and of "filial cannibalism" where adult Orthacanthus preyed upon juvenile Orthacanthus.[4] The genus Orthacanthus has been synonymized with Dittodus (Owen, 1867), Didymodus (Cope, 1883), and Diplodus (Agassiz, 1843).[3]
| Orthacanthus | |
|---|---|
 | |
| O. senckenbergianus fossil in Senckenberg Museum | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Chondrichthyes |
| Order: | †Xenacanthida |
| Family: | †Orthacanthidae Heyler and Poplin, 1989 |
| Genus: | †Orthacanthus Agassiz, 1843 |
| Species | |
| |
About 260 million years ago, Orthacanthus was the apex predator of freshwater swamps and bayous in Europe and North America.[4] Mature Orthacanthus reached nearly 3 meters (10 feet) in length.[4] Orthacanthus teeth have a minimum of three cusps, two principal cusps, and an intermediate cusp, where the principal cusps are variously serrated, with complex base morphology.[5][2] Additionally, Orthacanthus can be diagnosed by major transverse axes of proximal ends at a 45 degree angle to and often almost parallel to the labial margin of the base between the cusps.[2] Deformed teeth are characteristic of the xenacanth sharks and of Orthacanthus.[6]
Description and Paleobiology
Teeth

The larger teeth of Orthacanthus compressus and Orthacanthus texensis are differentiated by a more pronounced basal tubercle in O. compressus.[5] The basal tubercle of a typical tooth file is on the apical button of the underlying tooth.[6] The larger adult teeth of O. compressus have a wider rather than longer base, similar to O. texensis, and tend to have serrations on both carinae of each cusp, while the medial carinae of smaller adult teeth are not serrated.[5] The juvenile teeth of O. compressus are longer than wide, have a thinner base, and lack serrations, similar to O. platypternus teeth.[5]
Orthacanthus platypternus from the Craddock Bonebed shark layer in Texas, USA, shows evidence of resorption, and the equivalent of an "enamel pearl."[6] Some of the teeth specimens found at this location show evidence of resorption, which has not been previously observed in other faunas at the same location.[6] Where the superjacent basal tubercle is expected to be resorbed if the teeth were to undergo resorption, the apical button is resorbed instead.[6]
Sexual Dimorphism
The difference in characteristics between the large and small O. compressus adult teeth might indicate sexual dimorphism.[5]
The spines of O. platypternus showing 3 to 4 dentine layers are interpreted to be subadults or young adults, and are separated into two size classes where females have the largest spines in comparison to males, indicating sexual dimorphism.[7]
Dorsal Spine, Dentine, and Denticles
The dorsal spines of Orthacanthus platypternus from the Craddock Bone Bed in Texas, USA, preserve a highly vascularized wall mainly composed of centrifugally growing dentine (the outer layer of the wall of the spine) in a succession of inwardly growing dentine layers that line the pulp cavity.[7] These dentine layers are likely deposited periodically in accordance with seasonal variations in water temperature and food availability.[7] More specifically, the periodic nature of the dentine layer deposits could be due to variation in calcium phosphate deposition following the changes in water temperature.[8] Spines of individuals with 1-2 dentine layers are likely juveniles and result in the smallest sizes, whereas individuals showing at least 3-4 dentine layers result in two separate size classes.[7] The cross section is oval near the opening of the pulp cavity and circular/subtriangular in the distal part of the non-denticulated region and circular in the denticulated region.[7] The pulp cavity of the spine is filled with calcite, quartz, and opaque minerals.[7]
Occipital spine and denticles

The spine is superficially inserted in the skin, where it grows and moves from a deep position in the dermis where trabecular dentine forms, to a superficial location where centrifugally growing lamellar dentine forms.[8] The number of denticles per annual cycle vary with growth rate, and are independent dermal elements formed by the dermal papilla and secondarily attached by dentine to the spine proper.[8] The density of denticulation also varies with the growth rate of the occipital spine.[8] The ratio of length of denticulated region to total length of the spine changes throughout ontogeny.[8]
Historical information and discovery
Genera Orthacanthus and Pleuracanthus were founded by Agassiz in 1837 on isolated "ichthyodorulites" from the British Carboniferous System, and at the time were mistakenly thought of as the first indicators of Skates.[9] They were initially found throughout the United Kingdom in Dudley, Leeds, North Wales, Carluke, and Edinburh.[9] Three additional species from the Carboniferous formation of Ohio were described by Dr. Newberry. Teeth associated with Diplodus, a genus of sharks, was found in the Carboniferous slates of England in Stafford, Carluke, and Burdiehouse, and in Nova Scotia.[9] A well preserved impression fromm Ruppelsdorf, Bohemia was described by Goldfuss, while a separate paper published the same specimen under the name Xenacanthus Dechenii. One year later in 1849, Dr. Jordan mistakenly identified the same specimen as the remains of a fossil shark Triodus sessilis. This mistake was corrected and identified as Xenacanthus by Mr. Schnur.[9]
Classification
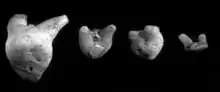

The teeth of Orthacanthus texensis and Orthacanthus platypternus from bonebeds from the Lower Permian of Texas, and the teeth of Orthacanthus compressus from the Upper Pennsylvanian of Nebraska and Dunkard Basin of the central Appalachians were used to determine the origin of O. texensis and O. platypternus.[5] It has been proposed that both O. texensis and O. platypternus could be derived from O. compressus, where juvenile features of O. compressus are retained in the adult teeth of O. platypternus via paedomorphosis, and the juvenile features of O. compressus teeth are observed in the adult teeth of O. texensis.[5]
Taxon Mimia sits outside of a clade that contains two monophyletic sister groups. The first monophyletic sister group defines a clade that includes the stem-group chondrichthyans which is visualized as a sister group of Doliodus and a large clade that comprises the cladodont sharks and Orthacanthus. The “cladodont sharks” plus Orthacanthus comprise two monophyletic sister groups: Orthacanthus and the Cladodoides and Tamiobatis on one side, and the Symmoriiformes on the other side. The second monophyletic sister group characterizes crown chondrichthyans which contains two monophyletic sister groups (Euchondrocephali on one side and Euselachii on the other side).[10]
Paleobiology

A 2013 analysis of oxygen and strontium isotope composition of the teeth and spines of Late Carboniferous and Early Permian shark taxa was performed to infer the hydrochemistry of their ambient water, thus contributing to the controversy between an obligate freshwater or euryhaline diadromous lifestyle.[11] Facies interpretations in the Permian of North America suggested that salinity tolerance of xenacanthids was restricted to near marine environments while only Orthacanthus can tolerate brackish water environments.[11] A study covering the morphology and histology of dorsal spines of Orthacanthus platypternus also reported that "The comparative analyses of the ontogenetic stages of the recorded specimens of O. platypternus and their distribution along different facies and localities indicate that this species was euryhaline, diadromous with a catadromous life-cycle which was strongly regulated by the semi-arid, seasonally dry tropical climate affecting western Pangaea during the Early Permian.[7]" The 2013 analysis provided evidence leaning towards an obligate freshwater lifestyle of the sharks from the studied Variscan European basins, and nonmarine ratios suggested teeth formation was influence by meteoric waves enriched by evaporation.[11] Euryhaline adaptation was not confirmed in the 2013 analysis.[11]
Predator-Prey relationship
Orthacanthus and Triodus have a predator-prey relationship where specifically there is predation of Orthacanthus on Triodus.[12] Cranial remains of specimens of both Orthacanthus and Triodus were from the Upper Carboniferous in Puertollano basin, Spain, and give evidence of this predator-prey relationship.[12] Numerous and well preserved cephalic elements of Triodus were associated with the cranial remains of Orthacanthus, and is explained by the inclusion of occipital spines of Triodus in the buccal cavity of Orthacanthus.[12] Additional evidence is the co-occurrence of one Orthacanthus spine with many Triodus spines, which likely penetrated the soft tissue and cartilage of the mouth of Orthacanthus, similarly to modern sharks that feed on stingrays where the spines of stingrays have been found within and around the buccal cavities of Carcharhinus, Galeocerdo, Negaprion and Sphyrna.
Examination of Orthacanthus coprolites from Canada by Aodhan O' Gogain et al revealed that in times of hardship, Orthacanthus was likely cannibalistic, as teeth from juvenile Orthacanthus were found within the coprolites of adults.[13][14] Orthacanthus had a catholic diet that consisted of actinopterygians, acanthodians, dipnoans, xenacanthids and tetrapods, based on analysis of coprolites and gut contents.[4] There have also been suspicions of filial cannibalism due to the presence of juvenile Orthacanthus teeth inside an Orthacanthus coprolite. The poop of Orthacanthus has a unique spiraling shape due to a corkscrew-shaped rectum.[4]
Paleoecology

The palaeobiogeographical distribution of O. platypternus suggests ontogenetic habitat partitioning.[7] Ontogenetic niche theory predicts that individuals may change their habitat or diet to maintain optimal growth rates or to improve trade-offs between mortality risk and growth.[15] While smaller individuals likely lived in shallower waters such as in small ponds and stream channels of the coastal plain, larger individuals likely lived in deeper water such as the fluvio-lacustrine (rivers and lakes) and marginal marine areas.[7]
The oldest known specimen of Orthacanthus, Diplodus problematicus was found in New Brunswick, Canada, from the Emsian, ranging between 407 and 393 million years ago.[3] Other specimens have been found in locations including the US, the United Kingdom, Poland, and France.[3]
References
| Wikimedia Commons has media related to Orthacanthus. |
- Hampe, O. "On the Dentition of Orthacanthus (Chondrichthyes, Xencanthida) Upper Carboniferous-Lower Permian." Palaont Z 62.3-4 (1988).
- Johnson, Gary D.; Thayer, David W. (2009). "Early Pennsylvanian Xenacanth Chondrichthyans from the Swisshelm Mountains, Arizona, USA". Acta Palaeontologica Polonica. 54 (4): 649–668. doi:10.4202/app.2008.0051. ISSN 0567-7920. S2CID 54093505.
- "PBDB". paleobiodb.org. Retrieved 2020-03-04.
- Gogáin, Aodhán Ó; Falcon-Lang, Howard J.; Carpenter, David K.; Miller, Randall F.; Benton, Michael J.; Pufahl, Peir K.; Ruta, Marcello; Davies, Thomas G.; Hinds, Steven J.; Stimson, Matthew R. (11 August 2016). "Data from: Fish and tetrapod communities across a marine to brackish salinity gradient in the Pennsylvanian (early Moscovian) Minto Formation of New Brunswick, Canada, and their palaeoecological and palaeogeographical implications" (PDF). Palaeontology. doi:10.1111/pala.12249. ISSN 1475-4983.
- Johnson, Gary D. (2012). "Possible origin of the xenacanth sharksOrthacanthus texensisandOrthacanthus platypternusin the Lower Permian of Texas, USA". Historical Biology. 24 (4): 369–379. doi:10.1080/08912963.2012.669128. ISSN 0891-2963. S2CID 128880622.
- Johnson, G. D. (2018). "Orthacanthus platypternus (Cope, 1883) (Chondrichthyes: Xenacanthiformes) teeth and other isolated vertebrate remains from a single horizon in the early Permian (Artinskian) Craddock Bonebed, lower Clear Fork Group, Baylor County, Texas, USA". Acta Geologica Polonica. 68 (3). doi:10.1515/agp-2018-0025 (inactive 2021-01-17). ISSN 0001-5709.CS1 maint: DOI inactive as of January 2021 (link)
- Beck, Kimberly; Soler-Gijon, Rodrigo; Carlucci, Jesse; Willis, Raymond (2014). "Morphology and histology of dorsal spines of the xenacanthid shark Orthacanthus platypternus from the Lower Permian of Texas, USA: palaeobiological and palaeoenvironmental implications". Acta Palaeontologica Polonica. doi:10.4202/app.00126.2014. ISSN 0567-7920. S2CID 53979047.
- Soler-Gijon, Rodrigo (1999). "Occipital spine ofOrthacanthus (Xenacanthidae, Elasmobranchii): Structure and growth". Journal of Morphology. 242 (1): 1–45. doi:10.1002/(sici)1097-4687(199910)242:1<1::aid-jmor2>3.0.co;2-9. ISSN 0362-2525. PMID 10493780.
- Lütken, Christian (1868). "II.—On Xenacanthus (Orthacanthus) Dechenii , Goldfuss. By ProfessorKner , of Vienna". Geological Magazine. 5 (50): 376–380. Bibcode:1868GeoM....5..376L. doi:10.1017/S0016756800205025. ISSN 0016-7568.
- Pradel, Alan; Tafforeau, Paul; Maisey, John G.; Janvier, Philippe (2011-09-27). "A New Paleozoic Symmoriiformes (Chondrichthyes) from the Late Carboniferous of Kansas (USA) and Cladistic Analysis of Early Chondrichthyans". PLOS ONE. 6 (9): e24938. Bibcode:2011PLoSO...624938P. doi:10.1371/journal.pone.0024938. ISSN 1932-6203. PMC 3181253. PMID 21980367.
- Fischer, Jan; Schneider, Jörg W.; Voigt, Silke; Joachimski, Michael M.; Tichomirowa, Marion; Tütken, Thomas; Götze, Jens; Berner, Ulrich (2013). "Oxygen and strontium isotopes from fossil shark teeth: Environmental and ecological implications for Late Palaeozoic European basins". Chemical Geology. 342: 44–62. Bibcode:2013ChGeo.342...44F. doi:10.1016/j.chemgeo.2013.01.022. ISSN 0009-2541.
- Soler-Gijón, Rodrigo (1995). "Evidence of predator-prey relationship in xenacanth sharks of the Upper Carboniferous (Stephanian C) from Puertollano; Spain". Geobios. 28: 151–156. doi:10.1016/s0016-6995(95)80104-9. ISSN 0016-6995.
- "Scientists just found out something disturbing about a massive ancient species of shark".
- "Unearthed: The cannibal sharks of a forgotten age".
- Ramirez, Matthew D.; Avens, Larisa; Seminoff, Jeffrey A.; Goshe, Lisa R.; Heppell, Selina S. (2017-02-16). "Growth dynamics of juvenile loggerhead sea turtles undergoing an ontogenetic habitat shift". Oecologia. 183 (4): 1087–1099. Bibcode:2017Oecol.183.1087R. doi:10.1007/s00442-017-3832-5. ISSN 0029-8549. PMID 28210809. S2CID 225674.