Rhodium(III) sulfide
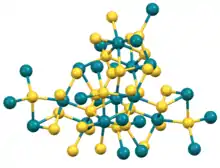
Rhodium(III) sulfide is the inorganic compound with the formula Rh2S3. It is an insoluble black solid, prepared by the heating a mixture of elemental rhodium and sulfur. Crystals can be grown by chemical vapor transport using bromine as the transporting agent. The structure consists of octahedral and tetrahedral Rh and S centers, respectively. No close Rh-Rh contacts are observed.[1] Rh2Se3 and Ir2S3 adopt the same structure as Rh2S3.
 | |
| Names | |
|---|---|
| IUPAC name
Rhodium(III) sulfide | |
| Other names
Rhodium sesquisulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.874 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Rh2S3 | |
| Molar mass | 301.99 g·mol−1 |
| Appearance | black solid |
| Density | 6.46 g/cm−3 |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Parthé, Erwin; Hohnke, Dieter K.; Hulliger, Fritz (1967). "New Structure Type with Octahedron Pairs for Rhodium(III) Sulfide, Rhodium(III) Selenide, and Iridium(III) Sulfide". Acta Crystallographica. 23: 832–40. doi:10.1107/S0365110X67003767.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.