Rhodochrosite
Rhodochrosite is a manganese carbonate mineral with chemical composition MnCO3. In its (rare) pure form, it is typically a rose-red color, but impure specimens can be shades of pink to pale brown. It streaks white, and its Mohs hardness varies between 3.5 and 4. Its specific gravity is between 3.5 and 3.7. It crystallizes in the trigonal system, and cleaves with rhombohedral carbonate cleavage in three directions. Crystal twinning often is present. It is transparent to translucent with refractive indices of nω=1.814 to 1.816, nε=1.596 to 1.598. It is often confused with the manganese silicate, rhodonite, but is distinctly softer. It is officially listed as one of the National symbols of Argentina.
| Rhodochrosite | |
|---|---|
 | |
| General | |
| Category | Carbonate minerals |
| Formula (repeating unit) | MnCO3 |
| Strunz classification | 5.AB.05 |
| Crystal system | Trigonal |
| Crystal class | Hexagonal scalenohedral (3m) H-M symbol: (3 2/m) |
| Space group | R3c |
| Unit cell | a = 4.777, c = 15.67 [Å]; Z = 6 |
| Identification | |
| Formula mass | 114.95 g/mol |
| Color | Pink, rose, rose-red, red, cherry-red, yellow, yellowish gray, gray, cinnamon-brown, white, may be banded; colourless to pale rose in transmitted light. |
| Crystal habit | Rhombohedral and scalenohedral crystals; also commonly bladed, columnar, stalactitic, botryoidal, granular or massive |
| Twinning | On {1012} as contact and lamellar |
| Cleavage | On {1011} perfect; parting on {1012} |
| Fracture | Uneven, conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 3.5–4 |
| Luster | Vitreous to pearly |
| Streak | White |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 3.7 |
| Optical properties | Uniaxial (-) |
| Refractive index | nω = 1.814–1.816 nε = 1.596–1.598 |
| Birefringence | δ = 0.218 |
| Pleochroism | weak |
| Ultraviolet fluorescence | None |
| References | [1][2][3] |
Rhodochrosite forms a complete solid solution series with iron carbonate (siderite). Calcium, (as well as magnesium and zinc, to a limited extent) frequently substitutes for manganese in the structure, leading to lighter shades of red and pink, depending on the degree of substitution. It is for this reason that the most common color encountered is pink.
Occurrence and discovery
Rhodochrosite occurs as a hydrothermal vein mineral along with other manganese minerals in low temperature ore deposits as in the silver mines of Romania where it was first found. Banded rhodochrosite is mined in Capillitas, Argentina.
It was first described in 1813 in reference to a sample from Cavnic, Maramureş, present-day Romania. According to Dimitrescu and Radulescu, 1966 and to Papp, 1997, this mineral was described for the first time in Sacaramb, Romania, not in Cavnic, Romania. The name is derived from the Greek word ῥοδόχρως meaning rose-colored.
Use
Its main use is as an ore of manganese, which is a key component of low-cost stainless steel formulations and certain aluminium alloys. Quality banded specimens are often used for decorative stones and jewelry. Due to its being relatively soft, and having perfect cleavage, it is very difficult to cut, and therefore rarely found faceted in jewelry.
Manganese carbonate is extremely destructive to the amalgamation process used in the concentration of silver ores, and were often discarded on the mine dump.
Culture
| ||
| ||
| ||
| ||
| ||
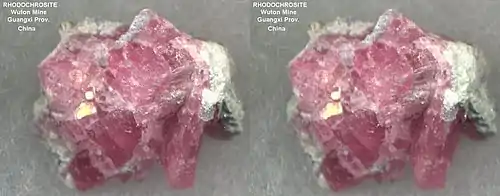
| Small Rhodochrosite specimen featured in a mineral kit, from Wuton mine, Guangxi prov, China. |
Rhodochrosite is Argentina's "national gemstone".[4][5] Colorado officially named rhodochrosite as its state mineral in 2002.[6]
It is sometimes called "Rosa del Inca", "Inca Rose" or Rosinca.[7]
Gallery
 "The Searchlight," Large red rhodochrosite from Sweet Home Mine, Alma, Colorado
"The Searchlight," Large red rhodochrosite from Sweet Home Mine, Alma, Colorado Rhodochrosite with fluorite, tetrahedrite and quartz
Rhodochrosite with fluorite, tetrahedrite and quartz Pink is the most common color of Rhodochrosite. Specimen mined near Silverton, Colorado
Pink is the most common color of Rhodochrosite. Specimen mined near Silverton, Colorado The Alma King is the largest known rhodochrosite crystal; it was found in the Sweet Home Mine near Alma, Colorado. It is on display in the Denver Museum of Nature and Science.
The Alma King is the largest known rhodochrosite crystal; it was found in the Sweet Home Mine near Alma, Colorado. It is on display in the Denver Museum of Nature and Science.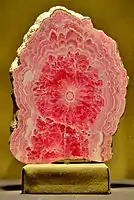
 The Alma Rose specimen from the Sweet Home Mine in Alma, CO. Located at the Rice Northwest Museum of Rocks and Minerals in Hillsboro, OR.
The Alma Rose specimen from the Sweet Home Mine in Alma, CO. Located at the Rice Northwest Museum of Rocks and Minerals in Hillsboro, OR.
See also
| Wikimedia Commons has media related to Rhodochrosite. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Rhodochrosite. |
References
- Handbook of Mineralogy
- Rhodochrosite data on Mindat
- Rhodochrosite data on Webmineral
- "Piedra nacional: la Rodocrosita" (in Spanish). Embassy of the Argentine Republic in the Colombian Republic. Retrieved 7 October 2013.
- Moreno, María (9 November 2002). "La piedra argentina". Página/12 (in Spanish). Retrieved 7 October 2013.
- "Colorado State Archives; Symbols & Emblems". Retrieved 2 February 2012.
- R. V. Dietrich (2005-07-16). "Rhodochrosite". Retrieved 2007-08-15.
- Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., ISBN 0-471-80580-7.