Seagrass
Seagrasses are the (only) flowering plants which grow in marine environments. There are about 60 species of fully marine seagrasses which belong to four families (Posidoniaceae, Zosteraceae, Hydrocharitaceae and Cymodoceaceae), all in the order Alismatales (in the class of monocotyledons).[1] Seagrasses evolved from terrestrial plants which recolonised the ocean 70 to 100 million years ago.

The name seagrass stems from the many species with long and narrow leaves, which grow by rhizome extension and often spread across large "meadows" resembling grassland; many species superficially resemble terrestrial grasses of the family Poaceae.
Like all autotrophic plants, seagrasses photosynthesize, in the submerged photic zone, and most occur in shallow and sheltered coastal waters anchored in sand or mud bottoms. Most species undergo submarine pollination and complete their life cycle underwater.
Seagrasses form dense underwater seagrass meadows which are among the most productive ecosystems in the world. They function as important carbon sinks and provide habitats and food for a diversity of marine life comparable to that of coral reefs.
Evolution
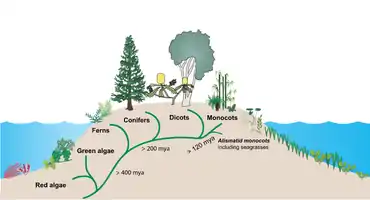
Terrestrial plants evolved perhaps as early as 450 million years ago from a group of green algae.[2] Seagrasses then evolved from terrestrial plants which migrated back into the ocean.[3][4] Between about 70 million and 100 million years ago, the three independent seagrass lineages (Hydrocharitaceae, Cymodoceaceae complex, and Zosteraceae) evolved from a single lineage of monocotyledonous flowering plants.[5]
Other plants that colonised the sea, such as salt marsh plants, mangroves, and marine algae, have more diverse evolutionary lineages. In spite of their low species diversity, seagrasses have succeeded in colonising the continental shelves of all continents except Antarctica.[6]
Taxonomy
| Family | Image | Genera | Description |
|---|---|---|---|
| Zosteraceae | The family Zosteraceae, also known as the seagrass family, includes two genera containing 14 marine species. It is found in temperate and subtropical coastal waters, with the highest diversity located around Korea and Japan. Species subtotal: | ||
 |
Phyllospadix | 6 species | |
 |
Zostera | 16 species | |
| Hydrocharitaceae | The family Hydrocharitaceae, also known as tape-grasses, include Canadian waterweed and frogbit. The family includes both fresh and marine aquatics, although of the sixteen genera currently recognised, only three are marine.[7] They are found throughout the world in a wide variety of habitats, but are primarily tropical. Species subtotal: | ||
 |
Enhalus | 1 species | |
 |
Halophila | 19 species | |
 |
Thalassia | 2 species | |
| Posidoniaceae | The family Posidoniaceae contains a single genus with two to nine marine species found in the seas of the Mediterranean and around the south coast of Australia. Species subtotal: 2 to 9 | ||
 |
Posidonia | 2 to 9 species | |
| Cymodoceaceae | The family Cymodoceaceae, also known as manatee-grass, includes only marine species.[8] Some taxonomists do not recognized this family. Species subtotal: | ||
 |
Amphibolis | 2 species | |
 |
Cymodocea | 4 species | |
 |
Halodule | 6 species | |
 |
Syringodium | 2 species | |
 |
Thalassodendron | 3 species | |
| Total species: | |||
Intertidal and subtidal seagrasses
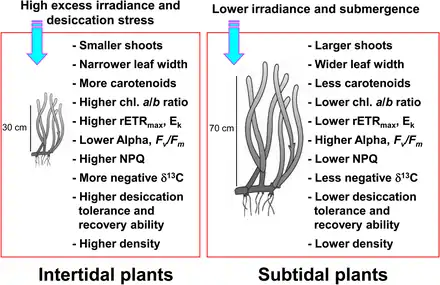
Seagrasses occurring in the intertidal and subtidal zones are exposed to highly variable environmental conditions due to tidal changes.[10][11] Seagrasses in the intertidal zone are regularly exposed to air and consequently experience extreme high and low temperatures, high photoinhibitory irradiance, and desiccation stress relative to subtidal seagrass.[11][12][13] Such extreme temperatures can lead to significant seagrass dieback when seagrasses are exposed to air during low tide.[14][15][16] Desiccation stress during low tide has been considered the primary factor limiting seagrass distribution at the upper intertidal zone.[17] Seagrasses residing the intertidal zone are usually smaller than those in the subtidal zone to minimize the effects of emergence stress.[18][15] Intertidal seagrasses also show light-dependent responses, such as decreased photosynthetic efficiency and increased photoprotection during periods of high irradiance and air exposure.[19][20]

In contrast, seagrasses in the subtidal zone adapt to reduced light conditions caused by light attenuation and scattering due to the overlaying water column and suspended particles.[22][23] Seagrasses in the deep subtidal zone generally have longer leaves and wider leaf blades than those in the shallow subtidal or intertidal zone, which allows more photosynthesis, in turn resulting in greater growth.[13] Seagrasses also respond to reduced light conditions by increasing chlorophyll content and decreasing the chlorophyll a/b ratio to enhance light absorption efficiency by using the abundant wavelengths efficiently.[24][25][26] As seagrasses in the intertidal and subtidal zones are under highly different light conditions, they exhibit distinctly different photoacclimatory responses to maximize photosynthetic activity and photoprotection from excess irradiance.
Seagrasses assimilate large amounts of inorganic carbon to achieve high level production.[27][28] Marine macrophytes, including seagrass, use both CO2 and HCO−
3 (bicarbonate) for photosynthetic carbon reduction.[29][30][31] Despite air exposure during low tide, seagrasses in the intertidal zone can continue to photosynthesize utilizing CO2 in the air.[32] Thus, the composition of inorganic carbon sources for seagrass photosynthesis probably varies between intertidal and subtidal plants. Because stable carbon isotope ratios of plant tissues change based on the inorganic carbon sources for photosynthesis,[33][34] seagrasses in the intertidal and subtidal zones may have different stable carbon isotope ratio ranges.
Seagrass microbiome
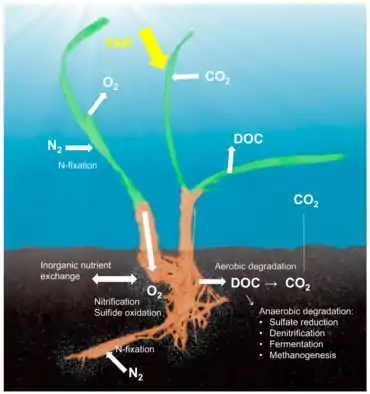
Seagrass holobiont
The concept of the holobiont, which emphasizes the importance and interactions of a microbial host with associated microorganisms and viruses and describes their functioning as a single biological unit,[37] has been investigated and discussed for many model systems, although there is substantial criticism of a concept that defines diverse host-microbe symbioses as a single biological unit.[38] The holobiont and hologenome concepts have evolved since the original definition,[39] and there is no doubt that symbiotic microorganisms are pivotal for the biology and ecology of the host by providing vitamins, energy and inorganic or organic nutrients, participating in defense mechanisms, or by driving the evolution of the host.[40] Although most work on host-microbe interactions has been focused on animal systems such as corals, sponges, or humans, there is a substantial body of literature on plant holobionts.[41] Plant-associated microbial communities impact both key components of the fitness of plants, growth and survival,[42] and are shaped by nutrient availability and plant defense mechanisms.[43] Several habitats have been described to harbor plant-associated microbes, including the rhizoplane (surface of root tissue), the rhizosphere (periphery of the roots), the endosphere (inside plant tissue), and the phyllosphere (total above-ground surface area).[35]
Seagrass meadows
Seagrass beds/meadows can be either monospecific (made up of a single species) or in mixed beds. In temperate areas, usually one or a few species dominate (like the eelgrass Zostera marina in the North Atlantic), whereas tropical beds usually are more diverse, with up to thirteen species recorded in the Philippines.
Seagrass beds are diverse and productive ecosystems, and can harbor hundreds of associated species from all phyla, for example juvenile and adult fish, epiphytic and free-living macroalgae and microalgae, mollusks, bristle worms, and nematodes. Few species were originally considered to feed directly on seagrass leaves (partly because of their low nutritional content), but scientific reviews and improved working methods have shown that seagrass herbivory is an important link in the food chain, feeding hundreds of species, including green turtles, dugongs, manatees, fish, geese, swans, sea urchins and crabs. Some fish species that visit/feed on seagrasses raise their young in adjacent mangroves or coral reefs.
Seagrasses trap sediment and slow down water movement, causing suspended sediment to settle out. Trapping sediment benefits coral by reducing sediment loads, improving photosynthesis for both coral and seagrass.[44]

 White-spotted puffers, often found in seagrass areas
White-spotted puffers, often found in seagrass areas


Although often overlooked, seagrasses provide a number of ecosystem services.[45][46] Seagrasses are considered ecosystem engineers.[47][4][3] This means that the plants alter the ecosystem around them. This adjusting occurs in both physical and chemical forms. Many seagrass species produce an extensive underground network of roots and rhizome which stabilizes sediment and reduces coastal erosion.[48] This system also assists in oxygenating the sediment, providing a hospitable environment for sediment-dwelling organisms.[47] Seagrasses also enhance water quality by stabilizing heavy metals, pollutants, and excess nutrients.[49][4][3] The long blades of seagrasses slow the movement of water which reduces wave energy and offers further protection against coastal erosion and storm surge. Furthermore, because seagrasses are underwater plants, they produce significant amounts of oxygen which oxygenate the water column. These meadows account for more than 10% of the ocean's total carbon storage. Per hectare, it holds twice as much carbon dioxide as rain forests and can sequester about 27.4 million tons of CO2 annually.[50] The storage of carbon is an essential ecosystem service as we move into a period of elevated atmospheric carbon levels. However, some climate change models suggest that some seagrasses will go extinct – Posidonia oceanica is expected to go extinct, or nearly so, by 2050.
Seagrass meadows provide food for many marine herbivores. Sea turtles, manatees, parrotfish, surgeonfish, sea urchins and pinfish feed on seagrasses. Many other smaller animals feed on the epiphytes and invertebrates that live on and among seagrass blades.[51] Seagrass meadows also provide physical habitat in areas that would otherwise be bare of any vegetation. Due to this three dimensional structure in the water column, many species occupy seagrass habitats for shelter and foraging. It is estimated that 17 species of coral reef fish spend their entire juvenile life stage solely on seagrass flats.[52] These habitats also act as a nursery grounds for commercially and recreationally valued fishery species, including the gag grouper (Mycteroperca microlepis), red drum, common snook, and many others.[53][54] Some fish species utilize seagrass meadows and various stages of the life cycle. In a recent publication, Dr. Ross Boucek and colleagues discovered that two highly sought after flats fish, the common snook and spotted sea trout provide essential foraging habitat during reproduction.[55] Sexual reproduction is extremely energetically expensive to be completed with stored energy; therefore, they require seagrass meadows in close proximity to complete reproduction.[55] Furthermore, many commercially important invertebrates also reside in seagrass habitats including bay scallops (Argopecten irradians), horseshoe crabs, and shrimp. Charismatic fauna can also be seen visiting the seagrass habitats. These species include West Indian manatee, green sea turtles, and various species of sharks. The high diversity of marine organisms that can be found on seagrass habitats promotes them as a tourist attraction and a significant source of income for many coastal economies along the Gulf of Mexico and in the Caribbean.
Relation to humans
Historically, seagrasses were collected as fertilizer for sandy soil. This was an important use in the Aveiro Lagoon, Portugal, where the plants collected were known as moliço.
In the early 20th century, in France and, to a lesser extent, the Channel Islands, dried seagrasses were used as a mattress (paillasse) filling - such mattresses were in high demand by French forces during World War I. It was also used for bandages and other purposes.
In February 2017, researchers found that seagrass meadows may be able to remove various pathogens from seawater. On small islands without wastewater treatment facilities in central Indonesia, levels of pathogenic marine bacteria – such as Enterococcus – that affect humans, fish and invertebrates were reduced by 50 percent when seagrass meadows were present, compared to paired sites without seagrass,[56] although this could be a detriment to their survival.[57]
Disturbances and threats
Natural disturbances, such as grazing, storms, ice-scouring and desiccation, are an inherent part of seagrass ecosystem dynamics. Seagrasses display a high degree of phenotypic plasticity, adapting rapidly to changing environmental conditions.
Seagrasses are in global decline, with some 30,000 km2 (12,000 sq mi) lost during recent decades. The main cause is human disturbance, most notably eutrophication, mechanical destruction of habitat, and overfishing. Excessive input of nutrients (nitrogen, phosphorus) is directly toxic to seagrasses, but most importantly, it stimulates the growth of epiphytic and free-floating macro- and micro-algae. This weakens the sunlight, reducing the photosynthesis that nourishes the seagrass and the primary production results.
Decaying seagrass leaves and algae fuels increasing algal blooms, resulting in a positive feedback. This can cause a complete regime shift from seagrass to algal dominance. Accumulating evidence also suggests that overfishing of top predators (large predatory fish) could indirectly increase algal growth by reducing grazing control performed by mesograzers, such as crustaceans and gastropods, through a trophic cascade.
Macroalgal blooms cause the decline and eradication of seagrasses. Known as nuisance species, macroalgae grow in filamentous and sheet-like forms and form thick unattached mats over seagrass, occurring as epiphytes on seagrass leaves. Eutrophication leads to the forming of a bloom, causing the attenuation of light in the water column, which eventually leads to anoxic conditions for the seagrass and organisms living in/around the plant(s). In addition to the direct blockage of light to the plant, benthic macroalgae have low carbon/nitrogen content, causing their decomposition to stimulate bacterial activity, leading to sediment resuspension, an increase in water turbidity and further light attenuation.[58][59]
When humans drive motor boats over shallow seagrass areas, sometimes the propeller blade can damage the seagrass.
The most-used methods to protect and restore seagrass meadows include nutrient and pollution reduction, marine protected areas and restoration using seagrass transplanting. Seagrass is not seen as resilient to the impacts of future environmental change.[60]
Restoration
In various locations, communities are attempting to restore seagrass beds that were lost to human action, including in the US states of Virginia,[61] Florida[62] and Hawaii,[63] as well as the United Kingdom.[64] Such reintroductions have been shown to improve ecosystem services.[65]
Dr. Fred Short of the University of New Hampshire developed a specialized transplant methodology known as "Transplanting Eelgrass Remotely with Frames" (TERF). This method involves using clusters of plants which are temporarily tied with degradable crepe paper unto a weighted frame of wire mesh. The method has already been tried out by Save The Bay.[66]
In 2001, Steve Granger, from the University of Rhode Island Graduate School of Oceanography used a boat-pulled sled that is able to deposit seeds below the sediment surface. Together with colleague Mike Traber (who developed a Knox gelatin matrix to encase the seeds in), they conducted a test planting at Narragansett Bay. They were able to plant a 400m² area in less than 2 hours.[67]
As of 2019 the Coastal Marine Ecosystems Research Centre of Central Queensland University has been growing seagrass for six years and has been producing seagrass seeds. They have been running trials in germination and sowing techniques.[68]
See also
- Alismatales
- Blue carbon
- Salt marsh
- Mangrove
- Ocean Data Viewer: contains the global distribution of seagrasses dataset
References
- Tomlinson and Vargo (1966). "On the morphology and anatomy of turtle grass, Thalassia testudinum (Hydrocharitaceae). I. Vegetative Morphology". Bulletin of Marine Science. 16: 748–761.
- Knauth, L. Paul; Kennedy, Martin J. (2009). "The late Precambrian greening of the Earth". Nature. 460 (7256): 728–732. Bibcode:2009Natur.460..728K. doi:10.1038/nature08213. PMID 19587681. S2CID 4398942.
- Orth; et al. (2006). "A global crisis for seagrass ecosystems". BioScience. 56 (12): 987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. hdl:10261/88476.
- Papenbrock, J (2012). "Highlights in seagrass' phylogeny, physiology, and metabolism: what makes them so species?". International Scholarly Research Network: 1–15.
- Les, D.H., Cleland, M.A. and Waycott, M. (1997) "Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily". Systematic Botany 22(3): 443–463.
- Orth, Robert J.; Carruthers, TIM J. B.; Dennison, William C.; Duarte, Carlos M.; Fourqurean, James W.; Heck, Kenneth L.; Hughes, A. Randall; Kendrick, Gary A.; Kenworthy, W. Judson; Olyarnik, Suzanne; Short, Frederick T.; Waycott, Michelle; Williams, Susan L. (2006). "A Global Crisis for Seagrass Ecosystems". BioScience. 56 (12): 987. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. ISSN 0006-3568.
- Christenhusz, Maarten J.M.; Byng, James W. (2016-05-20). "The number of known plants species in the world and its annual increase". Phytotaxa. 261 (3): 201. doi:10.11646/phytotaxa.261.3.1. ISSN 1179-3163.
- Waycott, Michelle; McMahon, Kathryn; Lavery, Paul (2014). A Guide to Southern Temperate Seagrasses. CSIRO Publishing. ISBN 9781486300150.
- Park, Sang Rul; Kim, Sangil; Kim, Young Kyun; Kang, Chang-Keun; Lee, Kun-Seop (2016). "Photoacclimatory Responses of Zostera marina in the Intertidal and Subtidal Zones". PLOS ONE. 11 (5): e0156214. Bibcode:2016PLoSO..1156214P. doi:10.1371/journal.pone.0156214. PMC 4881947. PMID 27227327.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Silva, J.; Santos, R. (2003). "Daily variation patterns in seagrass photosynthesis along a vertical gradient". Marine Ecology Progress Series. 257: 37–44. Bibcode:2003MEPS..257...37S. doi:10.3354/meps257037.
- Boese, Bruce L.; Robbins, Bradley D.; Thursby, Glen (2005). "Desiccation is a limiting factor for eelgrass (Zostera marina L.) distribution in the intertidal zone of a northeastern Pacific (USA) estuary". Botanica Marina. 48 (4). doi:10.1515/BOT.2005.037. S2CID 85105171.
- Durako, M. J.; Kunzelman, J. I.; Kenworthy, W. J.; Hammerstrom, K. K. (2003). "Depth-related variability in the photobiology of two populations of Halophila johnsonii and Halophila decipiens". Marine Biology. 142 (6): 1219–1228. doi:10.1007/s00227-003-1038-3. S2CID 85627116.
- Olivé, I.; Vergara, J. J.; Pérez-Lloréns, J. L. (2013). "Photosynthetic and morphological photoacclimation of the seagrass Cymodocea nodosa to season, depth and leaf position". Marine Biology. 160 (2): 285–297. doi:10.1007/s00227-012-2087-2. S2CID 86386210.
- Hemminga M. A. and Durate C. M. (2000) Seagrass ecology. Cambridge University Press.
- Seddon, S.; Cheshire, AC (2001). "Photosynthetic response of Amphibolis antarctica and Posidonia australis to temperature and desiccation using chlorophyll fluorescence". Marine Ecology Progress Series. 220: 119–130. Bibcode:2001MEPS..220..119S. doi:10.3354/meps220119.
- Hirst A, Ball D, Heislers S, Young P, Blake S, Coots A. Baywide Seagrass Monitoring Program, Milestone Report No. 2 (2008). Fisheries Victoria Technical Report No. 29, January 2009.
- Koch, Evamaria W. (2001). "Beyond Light: Physical, Geological, and Geochemical Parameters as Possible Submersed Aquatic Vegetation Habitat Requirements". Estuaries. 24 (1): 1–17. doi:10.2307/1352808. JSTOR 1352808. S2CID 85287808.
- Tanaka, Y.; Nakaoka, M. (2004). "Emergence stress and morphological constraints affect the species distribution and growth of subtropical intertidal seagrasses". Marine Ecology Progress Series. 284: 117–131. Bibcode:2004MEPS..284..117T. doi:10.3354/meps284117.
- Björk, M.; Uku, J.; Weil, A.; Beer, S. (1999). "Photosynthetic tolerances to desiccation of tropical intertidal seagrasses". Marine Ecology Progress Series. 191: 121–126. Bibcode:1999MEPS..191..121B. doi:10.3354/meps191121.
- Petrou, K.; Jimenez-Denness, I.; Chartrand, K.; McCormack, C.; Rasheed, M.; Ralph, PJ (2013). "Seasonal heterogeneity in the photophysiological response to air exposure in two tropical intertidal seagrass species" (PDF). Marine Ecology Progress Series. 482: 93–106. Bibcode:2013MEPS..482...93P. doi:10.3354/meps10229.
- Xu, Shaochun; Zhou, Yi; Wang, Pengmei; Wang, Feng; Zhang, Xiaomei; Gu, Ruiting (2016). "Salinity and temperature significantly influence seed germination, seedling establishment, and seedling growth of eelgrass Zostera marinaL". PeerJ. 4: e2697. doi:10.7717/peerj.2697. PMC 5119234. PMID 27896031.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Schwarz, A.-M.; Björk, M.; Buluda, T.; Mtolera, M.; Beer, S. (2000). "Photosynthetic utilisation of carbon and light by two tropical seagrass species as measured in situ". Marine Biology. 137 (5–6): 755–761. doi:10.1007/s002270000433. S2CID 86384408.
- Campbell, Stuart J.; McKenzie, Len J.; Kerville, Simon P.; Bité, Juanita S. (2007). "Patterns in tropical seagrass photosynthesis in relation to light, depth and habitat". Estuarine, Coastal and Shelf Science. 73 (3–4): 551–562. Bibcode:2007ECSS...73..551C. doi:10.1016/j.ecss.2007.02.014.
- Lee, Kun-Seop; Dunton, Kenneth H. (1997). "Effect of in situ light reduction on the maintenance, growth and partitioning of carbon resources in Thalassia testudinum banks ex König". Journal of Experimental Marine Biology and Ecology. 210: 53–73. doi:10.1016/S0022-0981(96)02720-7.
- Longstaff, B.J; Dennison, W.C (1999). "Seagrass survival during pulsed turbidity events: The effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis". Aquatic Botany. 65 (1–4): 105–121. doi:10.1016/S0304-3770(99)00035-2.
- Collier, CJ; Lavery, PS; Ralph, PJ; Masini, RJ (2008). "Physiological characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability". Marine Ecology Progress Series. 353: 65–79. Bibcode:2008MEPS..353...65C. doi:10.3354/meps07171.
- Lee, Kun-Seop; Park, Sang Rul; Kim, Young Kyun (2007). "Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review". Journal of Experimental Marine Biology and Ecology. 350 (1–2): 144–175. doi:10.1016/j.jembe.2007.06.016.
- Nayar, S.; Collings, G.J.; Miller, D.J.; Bryars, S.; Cheshire, A.C. (2009). "Uptake and resource allocation of inorganic carbon by the temperate seagrasses Posidonia and Amphibolis". Journal of Experimental Marine Biology and Ecology. 373 (2): 87–95. doi:10.1016/j.jembe.2009.03.010.
- Beer, Sven (1989). "Photosynthesis and photorespiration of marine angiosperms". Aquatic Botany. 34 (1–3): 153–166. doi:10.1016/0304-3770(89)90054-5.
- Larkum AWD, James PL. Towards a model for inorganic carbon uptake in seagrasses involving carbonic anhydrase. In Kuo J, Phillips RC, Walker DI, Kirkman H, editors. Seagrass biology: Proceedings of an International Workshop. Nedlands: The University of Western Australia; 1996. pp. 191–196.
- Beer, Sven; Rehnberg, Jon (1997). "The acquisition of inorganic carbon by the seagrass Zostera marina". Aquatic Botany. 56 (3–4): 277–283. doi:10.1016/S0304-3770(96)01109-6.
- Silva, João; Santos, Rui; Calleja, Maria Ll.; Duarte, Carlos M. (2005). "Submerged versus air-exposed intertidal macrophyte productivity: From physiological to community-level assessments". Journal of Experimental Marine Biology and Ecology. 317: 87–95. doi:10.1016/j.jembe.2004.11.010.
- O'Leary, Marion H. (1988). "Carbon Isotopes in Photosynthesis". BioScience. 38 (5): 328–336. doi:10.2307/1310735. JSTOR 1310735.
- Raven, John A.; Johnston, Andrew M.; Kübler, Janet E.; Korb, Rebecca; McInroy, Shona G.; Handley, Linda L.; Scrimgeour, Charlie M.; Walker, Diana I.; Beardall, John; Vanderklift, Mathew; Fredriksen, Stein; Dunton, Kenneth H. (2002). "Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses". Functional Plant Biology. 29 (3): 355–378. doi:10.1071/PP01201. PMID 32689482.
- Ugarelli, K., Chakrabarti, S., Laas, P. and Stingl, U. (2017) "The seagrass holobiont and its microbiome". Microorganisms, 5(4): 81. doi:10.3390/microorganisms5040081.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Tarquinio, F., Hyndes, G.A., Laverock, B., Koenders, A. and Säwström, C. (2019) "The seagrass holobiont: understanding seagrass-bacteria interactions and their role in seagrass ecosystem functioning". FEMS microbiology letters, 366(6): fnz057. doi:10.1093/femsle/fnz057.
- Margulis, Lynn (1991) "Symbiogenesis and Symbionticism". In: Symbiosis as a Source of Evolutionary Innovation; Margulis, L., Fester, R.(Eds.), Cambridge MIT Press. ISBN 9780262132695.
- Douglas, A.E.; Werren, J.H. (2016) "Holes in the Hologenome: Why Host-Microbe Symbioses Are Not Holobionts". mBio, 7: e02099-15. doi:10.1128/mBio.02099-15.
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.G.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A.; et al. Getting the Hologenome Concept Right: An Eco-Evolutionary Framework for Hosts and Their Microbiomes. mSystems 2016, 1, e00028-16. doi:10.1128/mSystems.00028-16.
- Rosenberg, E. and Zilber-Rosenberg, I. (2016) "Microbes drive evolution of animals and plants: the hologenome concept". MBio, 7(2). doi:10.1128/mBio.01395-15.
- Zilber-Rosenberg, I. and Rosenberg, E. (2008) "Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution". FEMS Microbiology Reviews, 32(5): 723–735. doi:10.1111/j.1574-6976.2008.00123.x.
- Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. and Dufresne, A. (2015) "The importance of the microbiome of the plant holobiont". New Phytologist, 206(4): 1196-1206. doi:10.1111/nph.13312.
- Sánchez-Cañizares, C., Jorrín, B., Poole, P.S. and Tkacz, A. (2017) "Understanding the holobiont: the interdependence of plants and their microbiome". Current Opinion in Microbiology, 38: 188–196. doi:10.1016/j.mib.2017.07.001.
- Seagrass-Watch: What is seagrass? Retrieved 2012-11-16.
- Nordlund, Lina; Koch, Evamaria W.; Barbier, Edward B.; Creed, Joel C. (2016-10-12). Reinhart, Kurt O. (ed.). "Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions". PLOS ONE. 11 (10): e0163091. Bibcode:2016PLoSO..1163091M. doi:10.1371/journal.pone.0163091. ISSN 1932-6203. PMC 5061329. PMID 27732600.
- United Nations Environment Programme (2020). Out of the blue: The value of seagrasses to the environment and to people. UNEP, Nairobi. https://www.unenvironment.org/resources/report/out-blue-value-seagrasses-environment-and-people
- Jones, Clive G.; Lawton, John H.; Shachak, Moshe (1994). "Organisms as ecosystem engineers". Oikos. 69 (3): 373–386. doi:10.2307/3545850. JSTOR 3545850.
- Grey, William; Moffler, Mark (1987). "Flowering of the seagrass Thalassia testudinum (Hydrocharitacea) in the Tampa Bay, Florida area". Aquatic Botany. 5: 251–259. doi:10.1016/0304-3770(78)90068-2.
- Darnell, Kelly; Dunton, Kenneth (2016). "Reproductive phenology of the subtropical seagrasses Thalassia testudinum (Turtle grass) and Halodule wrightii (Shoal grass) in the northwest Gulf of Mexico". Botanica Marina. 59 (6): 473–483. doi:10.1515/bot-2016-0080. S2CID 88685282.
- Macreadie, P. I.; Baird, M. E.; Trevathan-Tackett, S. M.; Larkum, A. W. D.; Ralph, P. J. (2013). "Quantifying and modelling the carbon sequestration capacity of seagrass meadows". Marine Pollution Bulletin. 83 (2): 430–439. doi:10.1016/j.marpolbul.2013.07.038. PMID 23948090.
- https://myfwc.com/research/habitat/seagrasses/information/faq/#:~:text=Expand%2FCollapse%20What%20animals%20eat,on%20and%20among%20seagrass%20blades.
- Nagelkerken, I.; Roberts, C. M.; van der Velde, G.; Dorenbosch, M.; van Riel, M. C.; Cocheret de la Morinière, E.; Nienhuis, P. H. (2002). "How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale". Marine Ecology Progress Series. 244: 299–305. Bibcode:2002MEPS..244..299N. doi:10.3354/meps244299.
- Nordlund, L. M.; Unsworth, R. K. F.; Gullstrom, M.; Cullen-Unsworth, L. C. (2018). "Global significance of seagrass fishery activity". Fish and Fisheries. 19 (3): 399–412. doi:10.1111/faf.12259.
- Unsworth, R. K. F.; Nordlund, L. M.; Cullen-Unsworth, L. C. (2019). "Seagrass meadows support global fisheries production". Conserv Lett. e12566: e12566. doi:10.1111/conl.12566.
- Boucek, R. E.; Leone, E.; Bickford, J.; Walters-Burnsed, S.; Lowerre-Barbieri, S. (2017). "More than just a spawning location: Examining fine-scale s[ace use of two estuarine fish species at a spawning aggregation site". Frontiers in Marine Science (4): 1–9.
- Byington, Cara (2017-02-17). "New Science Shows Seagrass Meadows Suppress Pathogens". Nature.org. NatureNet Fellows for Cool Green Science. Retrieved 17 February 2017.
- Jones, BJ; Cullen-Unsworth, L. C.; Unsworth, R. K. F. (2018). "Tracking Nitrogen Source Using δ15N Reveals Human and Agricultural Drivers of Seagrass Degradation across the British Isles". Frontiers in Plant Science. 9: 133. doi:10.3389/fpls.2018.00133. PMC 5808166. PMID 29467789.
- McGlathery, KJ (2001). "Macroalgal blooms contribute to the decline of seagrass in nutrient‐enriched coastal waters" (PDF). Journal of Phycology. 37 (4): 453–456. doi:10.1046/j.1529-8817.2001.037004453.x. S2CID 38983997.
- Fox SE, YS Olsen and AC Spivak (2010) "Effects of bottom-up and top-down controls and climate change on estuarine macrophyte communities and the ecosystem services they provide" In: PF Kemp (Ed) Eco-DAS Symposium Proceedings, ALSO, Chapter 8: 129–145.
- Unsworth, Richard K.F.; Collier, Catherine J.; Waycott, Michelle; McKenzie, Len J.; Cullen-Unsworth, Leanne C. (2015). "A framework for the resilience of seagrass ecosystems". Marine Pollution Bulletin. 100 (1): 34–46. doi:10.1016/j.marpolbul.2015.08.016. PMID 26342389.
- "Eelgrass Restoration | The Nature Conservancy in Virginia". www.nature.org. Retrieved 2018-08-06.
- "Seagrass Restoration". myfwc.com. Retrieved 2018-08-06.
- "Seagrass Restoration Initiative – Malama Maunalua". www.malamamaunalua.org. Retrieved 2018-08-06.
- Unsworth, Richard K. F.; McKenzie, Len J.; Collier, Catherine J.; Cullen-Unsworth, Leanne C.; Duarte, Carlos M.; Eklöf, Johan S.; Jarvis, Jessie C.; Jones, Benjamin L.; Nordlund, Lina M. (2019-08-01). "Global challenges for seagrass conservation". Ambio. 48 (8): 801–815. doi:10.1007/s13280-018-1115-y. ISSN 1654-7209. PMC 6541581. PMID 30456457.
- van Katwijk, Marieke M.; Thorhaug, Anitra; Marbà, Núria; Orth, Robert J.; Duarte, Carlos M.; Kendrick, Gary A.; Althuizen, Inge H. J.; Balestri, Elena; Bernard, Guillaume (2015-11-25). "Global analysis of seagrass restoration: the importance of large-scale planting". Journal of Applied Ecology. 53 (2): 567–578. doi:10.1111/1365-2664.12562. ISSN 0021-8901.
- Restoration Methods
- Restoration Methods
- Mackay, Jacquie; Stünzner, Inga (24 October 2019). "Seagrass nursery in central Queensland could offset carbon emissions". ABC News. Australian Broadcasting Corporation. Retrieved 24 October 2019.
One flower can produce 15 seeds, and one seed planted in the right conditions can create a hectare of seagrass.
Further references
- den Hartog, C. 1970. The Sea-grasses of the World. Verhandl. der Koninklijke Nederlandse Akademie van Wetenschappen, Afd. Natuurkunde, No. 59(1).
- Duarte, Carlos M. and Carina L. Chiscano “Seagrass biomass and production: a reassessment” Aquatic Botany Volume 65, Issues 1–4, November 1999, Pages 159–174.
- Green, E.P. & Short, F.T.(eds). 2003. World Atlas of Seagrasses. University of California Press, Berkeley, CA. 298 pp.
- Hemminga, M.A. & Duarte, C. 2000. Seagrass Ecology. Cambridge University Press, Cambridge. 298 pp.
- Hogarth, Peter The Biology of Mangroves and Seagrasses (Oxford University Press, 2007)
- Larkum, Anthony W.D., Robert J. Orth, and Carlos M. Duarte (Editors) Seagrasses: Biology, Ecology and Conservation (Springer, 2006)
- Orth, Robert J. et al. "A Global Crisis for Seagrass Ecosystems" BioScience December 2006 / Vol. 56 No. 12, Pages 987–996.
- Short, F.T. & Coles, R.G.(eds). 2001. Global Seagrass Research Methods. Elsevier Science, Amsterdam. 473 pp.
- A.W.D. Larkum, R.J. Orth, and C.M. Duarte (eds). Seagrass Biology: A Treatise. CRC Press, Boca Raton, FL, in press.
- A. Schwartz; M. Morrison; I. Hawes; J. Halliday. 2006. Physical and biological characteristics of a rare marine habitat: sub-tidal seagrass beds of offshore islands. Science for Conservation 269. 39 pp.
- Waycott, M, McMahon, K, & Lavery, P 2014, A guide to southern temperate seagrasses, CSIRO Publishing, Melbourne
External links
- Cullen-Unsworth, Leanne C.; Unsworth, Richard (2018-08-03). "A call for seagrass protection". Science. 361 (6401): 446–448. Bibcode:2018Sci...361..446C. doi:10.1126/science.aat7318 (inactive 2021-01-17). ISSN 0036-8075. PMID 30072524.CS1 maint: DOI inactive as of January 2021 (link)
- Project Seagrass - Charity advancing the conservation of seagrass through education, influence, research and action
- SeagrassSpotter - Citizen Science project raising awaress for seagrass meadows and mapping their locations
- Seagrass and Seagrass Beds overview from the Smithsonian Ocean Portal
- Nature Geoscience article describing the locations of the seagrass meadows around the world
- Seagrass-Watch - the largest scientific, non-destructive, seagrass assessment and monitoring program in the world
- Seagrass Ecosystem Research Group at Swansea University - Inter-disciplinary marine research for conservation
- Restore-A-Scar - a non-profit campaign to restore seagrass meadows damaged by boat props
- SeagrassNet - global seagrass monitoring program
- The Seagrass Fund at The Ocean Foundation
- Taxonomy of seagrasses
- World Seagrass Association
- SeagrassLI
- Seagrass Science and Management in the South China Sea and Gulf of Thailand
- Marine Ecology (December 2006) - special issue on seagrasses
- Cambodian Seagrasses
- Seagrass Productivity - COST Action ES0906
- Fisheries Western Australia - Seagrass Fact Sheet

