Semipermeable membrane
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis—or occasionally by more specialized processes of facilitated diffusion, passive transport or active transport. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials thicker than a membrane are also semipermeable. One example of this is the thin film on the inside of the egg. Note that a semipermeable membrane is not the same as a selectively permeable membrane. Semipermeable membrane describes a membrane that allows some particles to pass through (by size), whereas the selectively permeable membrane will choose what passes through (size is not a factor).
.svg.png.webp)
Biological membranes
An example of a biological semi-permeable membrane is the lipid bilayer, on which is based on the plasma membrane that surrounds all biological cells. A group of phospholipids (consisting of a phosphate head and two fatty acid tails) arranged into a double layer, the phospholipid bilayer is a semipermeable membrane that is very specific in its permeability. The hydrophilic phosphate heads are in the outside layer and exposed to the water content outside and within the cell. The hydrophobic tails are the layer hidden in the inside of the membrane. The phospholipid bilayer is most permeable to small, uncharged solutes. Protein channels float through the phospholipids, and, collectively, this model is known as the fluid mosaic model. Aquaporins are protein channel pores permeable to H2O water.
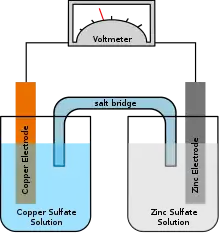
Reverse osmosis
The diffusion of water through a selectively permeable membrane is called osmosis. This allows only certain particles to go through including water and leaving behind the solutes including salt and other contaminants. In the process of reverse osmosis, thin film composite membranes (TFC or TFM) are used. These are semipermeable membranes manufactured principally for use in water purification or desalination systems. They also have use in chemical applications such as batteries and fuel cells. In essence, a TFC material is a molecular sieve constructed in the form of a film from two or more layered materials. Prof. Sidney Loeb and Srinivasa Sourirajan invented the first practical synthetic semi-permeable membrane.[1] Membranes used in reverse osmosis are, in general, made out of polyamide, chosen primarily for its permeability to water and relative impermeability to various dissolved impurities including salt ions and other small molecules that cannot be filtered. Another example of a semipermeable membrane is dialysis tubing.
Role in cellular communication
The semipermeable membrane is pertinent to cellular communication. A cell membrane consists of proteins and phospholipids.[2] Signaling molecules send chemical messages to the proteins in the cell membrane. The signaling molecules bind to proteins, which alters the protein structure.[3] A change in the protein structure initiates a signalling cascade.[3] An example of a technique that leverages membrane-based are tissue & cellular preservation technologies which show that adherent cells such as stem cells[4] and myoblasts[5] have better outcomes than non-adherent cells due to persistent signalling before and after preservation.[6]
Other types
Other types of semipermeable membranes are cation exchange membrane (CEM), charge mosaic membrane (CMM), bipolar membrane (BPM), anion exchange membrane (AEM) alkali anion exchange membrane (AAEM) and proton exchange membrane (PEM).
References
- , Sidney, Loeb & Sourirajan Srinivasa, "High flow porous membranes for separating water from saline solutions"
- Friedl, Sarah. "Semipermeable Membranes' Role in Cell Communication - Video & Lesson Transcript". Study.com. Retrieved 6 April 2017.
- Wood, David. "Semipermeable Membrane: Definition & Overview - Video & Lesson Transcript". Study.com. Retrieved 6 April 2017.
- Sambu, S.; Xu, X.; Schiffter, H. A.; Cui, Z. F.; Ye, H. (2011). "RGDS-Fuctionalized Alginates Improve the Survival Rate of Encapsulated Embryonic Stem Cells During Cryopreservation". Cryoletters.
- Ahmad, Hajira F.; Sambanis, Athanassios (2013). "Cryopreservation effects on recombinant myoblasts encapsulated in adhesive alginate hydrogels". Acta Biomaterialia. 9 (6): 6814–6822. doi:10.1016/j.actbio.2013.03.002. PMC 3664510. PMID 23499987.
- Hashemi, Maryam; Kalalinia, Fatemeh (15 December 2015). "Application of encapsulation technology in stem cell therapy". Life Sciences. 143: 139–146. doi:10.1016/j.lfs.2015.11.007. PMID 26556151.
Further reading
- Koros, W. J.; Ma, Y. H.; Shimidzu, T. (1 January 1996). "Terminology for membranes and membrane processes (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68 (7): 1479–1489. doi:10.1351/pac199668071479. S2CID 97076769. See this document for definitions of penetrant (permeant), synthetic (artificial) membrane, and anion-exchange membrane.
- Rozendal, R. A.; Sleutels, T. H. J. A.; Hamelers, H. V. M.; Buisman, C. J. N. (June 2008). "Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater". Water Science and Technology. 57 (11): 1757–1762. doi:10.2166/wst.2008.043. PMID 18547927.
- "High Flow Porous Membranes for Separating Water from Saline Solutions US 3133132 A". 12 May 1964. Retrieved 22 April 2014.
External links
- The European Membrane House, a non-profit international association created to continue the work of the network and parternships developed in NanoMemPro, an earlier EU-funded European network of membrane researchers.
- Short, non-scholarly WiseGeek article, "What is a Semipermeable Membrane.