Vanadium redox battery
The vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery that employs vanadium ions in different oxidation states to store chemical potential energy.[5] The vanadium redox battery exploits the ability of vanadium to exist in solution in four different oxidation states, and uses this property to make a battery that has just one electroactive element instead of two.[6] For several reasons, including their relative bulkiness, most vanadium batteries are currently used for grid energy storage, i.e., attached to power plants or electrical grids.
| Specific energy | 10–20 Wh/kg (36–72 J/g) |
|---|---|
| Energy density | 15–25 Wh/L (54–65 kJ/L) |
| Charge/discharge efficiency | 75–80%<.[1][2] |
| Time durability | 20-30 years |
| Cycle durability | >12,000-14,000 cycles [3] |
| Nominal cell voltage | 1.15–1.55 V |


The possibility of creating a vanadium flow battery was explored by Pissoort in the 1930s,[7] NASA researchers in the 1970s, and Pellegri and Spaziante in the 1970s,[8] but none of them were successful in demonstrating the technology. The first successful demonstration of the all-vanadium redox flow battery which employed vanadium in a solution of sulfuric acid in each half was by Maria Skyllas-Kazacos at the University of New South Wales in the 1980s.[9] Her design used sulfuric acid electrolytes, and was patented by the University of New South Wales in Australia in 1986.[2]
Numerous companies and organizations are involved in funding and developing vanadium redox batteries.
Advantages over other battery types
The main advantages of the vanadium redox battery are that it can offer almost unlimited energy capacity simply by using larger electrolyte storage tanks; it can be left completely discharged for long periods with no ill effects; if the electrolytes are accidentally mixed, the battery suffers no permanent damage; a single state of charge between the two electrolytes avoids the capacity degradation due to a single cell in non-flow batteries; the electrolyte is aqueous and inherently safe and non-flammable;[10] and the generation 3 formulation using a mixed acid solution developed by the Pacific Northwest National Laboratory operates over a wider temperature range[11] allowing for passive cooling.[12] VRFBs can be used at depth of discharge (DOD) around 90% and more, i.e. deeper DODs than solid-state batteries (e.g. lithium-based and sodium-based batteries, which are usually specified with DOD=80%). In addition, VRFBs exhibit very long cycle lives: most producers specify cycle durability in excess of 15,000-20,000 charge/discharge cycles. These values are far beyond the cycle lives of solid-state batteries, which is usually in the order of 4,000-5,000 charge/discharge cycles. Consequently, the levelized cost of energy (LCOE, i.e. the system cost divided by the usable energy, the cycle life, and round-trip efficiency) of present VRFB systems is typically in the order of a few tens of $ cents or € cents, much lower than the LCOEs of equivalent solid-state batteries and close to the targets of $0.05 and €0.05, stated by the US Department of Energy and the European Commission Strategic Energy Technology (SET) Plan, respectively.[13]
Disadvantages from other battery types
The main disadvantages of vanadium redox technology are a relatively poor energy-to-volume ratio compared to standard storage batteries, and relatively poor round trip efficiency. Furthermore, the aqueous electrolyte makes the battery heavy and therefore only useful for stationary applications. Another disadvantage is the relatively high toxicity of oxides of vanadium (see vanadium § Safety).
Operation
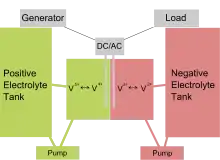
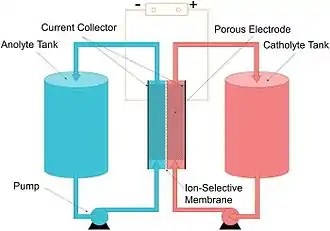
A vanadium redox battery consists of an assembly of power cells in which the two electrolytes are separated by a proton exchange membrane. The electrodes in a VRB cell are carbon based; the most common types being carbon felt, carbon paper, carbon cloth, and graphite felt. Recently, carbon nanotube based electrodes have gained marked interest from the scientific community.[14][15][16] Both electrolytes are vanadium-based, the electrolyte in the positive half-cells contains VO2+ and VO2+ ions, the electrolyte in the negative half-cells, V3+ and V2+ ions. The electrolytes may be prepared by any of several processes, including electrolytically dissolving vanadium pentoxide (V2O5) in sulfuric acid (H2SO4). The solution remains strongly acidic in use.
In vanadium flow batteries, both half-cells are additionally connected to storage tanks and pumps so that very large volumes of the electrolytes can be circulated through the cell. This circulation of liquid electrolytes is somewhat cumbersome and does restrict the use of vanadium flow batteries in mobile applications, effectively confining them to large fixed installations.
When the vanadium battery is being charged, the VO2+ ions in the positive half-cell are converted to VO2+ ions when electrons are removed from the positive terminal of the battery. Similarly in the negative half-cell, electrons are introduced converting the V3+ ions into V2+. During discharge this process is reversed and results in a typical open-circuit voltage of 1.41 V at 25 °C.
Other useful properties of vanadium flow batteries are their very fast response to changing loads and their extremely large overload capacities. Studies by the University of New South Wales have shown that they can achieve a response time of under half a millisecond for a 100% load change, and allowed overloads of as much as 400% for 10 seconds. The response time is mostly limited by the electrical equipment. Unless specifically designed for colder or warmer climates, most sulfuric acid-based vanadium batteries only work between about 10 and 40 °C. Below that temperature range, the ion-infused sulfuric acid crystallizes.[17] Round trip efficiency in practical applications is around 65–75 %.[18]
Proposed improvements
Second generation[19] vanadium redox batteries (vanadium/bromine) may approximately double the energy density and increase the temperature range in which the battery can operate. The vanadium/bromine and other vanadium based systems also reduce the cost of vanadium redox batteries by replacing the vanadium at the positive or negative electrolyte by cheaper alternatives such as cerium.[20]
Specific energy and energy density
Current production vanadium redox batteries achieve a specific energy of about 20 Wh/kg (72 kJ/kg) of electrolyte. More recent research at UNSW indicates that the use of precipitation inhibitors can increase the density to about 35 Wh/kg (126 kJ/kg), with even higher densities made possible by controlling the electrolyte temperature. This specific energy is quite low compared to other rechargeable battery types (e.g., lead–acid, 30–40 Wh/kg (108–144 kJ/kg); and lithium ion, 80–200 Wh/kg (288–720 kJ/kg)).
Applications
The extremely large capacities possible from vanadium redox batteries make them well suited to use in large power storage applications such as helping to average out the production of highly variable generation sources such as wind or solar power, helping generators cope with large surges in demand or leveling out supply/demand at a transmission constrained region.
The limited self-discharge characteristics of vanadium redox batteries make them useful in applications where the batteries must be stored for long periods of time with little maintenance while maintaining a ready state. This has led to their adoption in some military electronics, such as the sensor components of the GATOR mine system. Their ability to fully cycle and stay at 0% state of charge makes them suitable for solar + storage applications where the battery must start each day empty and fill up depending upon the load and weather. Lithium ion batteries, for example, are typically damaged when they are allowed to discharge below 20% state of charge, so they typically only operate between about 20% and 100%, meaning they are only using 80% of their nameplate capacity.[21]
Their extremely rapid response times also make them superbly well suited to uninterruptible power supply (UPS) type applications, where they can be used to replace lead–acid batteries and even diesel generators. Also the fast response time makes them well-suited for frequency regulation. Also, these capabilities make vanadium redox batteries an effective "all-in-one" solution for microgrids that depend on reliable operations, frequency regulation and have a need for load shifting (from either high renewable penetration, a highly variable load or desire to optimize generator efficiency through time-shifting dispatch).
Largest vanadium grid batteries
| Name | Commissioning date | Energy (MWh) | Power (MW) | Duration (hours) | Country |
|---|---|---|---|---|---|
| Minami Hayakita Substation[22][23] | December 2015 | 60 | 15 | 4 | Japan |
| Pfinztal, Baden-Württemberg[24][25][26] | September 2019 | 20 | 2 | 10 | Germany |
| Woniushi, Liaoning[27][28] | 10 | 5 | 2 | China | |
| Tomamae Wind Farm[29] | 2005 | 6 | 4 | 1:30 | Japan |
| Zhangbei Project[30] | 2016 | 8 | 2 | 4 | China |
| SnoPUD MESA 2 Project [31][32] | March 2017 | 8 | 2 | 4 | USA |
| San Miguel Substation[33] | 2017 | 8 | 2 | 4 | USA |
| Pullman Washington[34] | April 2015 | 4 | 1 | 4 | USA |
A 200 MW, 800 MWh (4 hours) vanadium redox battery is under construction in China; it was expected to be completed by 2018[35] and its 250 kW/ 1MWh first stage was in operation in late 2018[36]
Companies funding or developing vanadium redox batteries
Companies include UniEnergy Technologies,[37] StorEn Technologies,[38][39] and Ashlawn Energy[40] in the United States; Renewable Energy Dynamics Technology[41] and VoltStorage[42] in Europe; Prudent Energy in China;[43] Australian Vanadium in Australia.[44]
See also
| Wikimedia Commons has media related to Vanadium redox batteries. |
References
- Vanadium Battery Group University of New South Wales
- M. Skyllas-Kazacos, M. Rychcik and R. Robins, in AU Patent 575247 (1986), to Unisearch Ltd.
- Electricity Storage and Renewables: Costs and Markets to 2030. IRENA (2017), Electricity Storage and Renewables: Costs and Markets to 2030, International Renewable Energy Agency, Abu Dhabi.
- Qi, Zhaoxiang; Koenig, Gary M. (July 2017). "Review Article: Flow battery systems with solid electroactive materials". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena. 35 (4): 040801. doi:10.1116/1.4983210. ISSN 2166-2746.
- Laurence Knight (14 June 2014). "Vanadium: The metal that may soon be powering your neighbourhood". BBC. Retrieved 2 March 2015.
- Alotto, P.; Guarnieri, M.; Moro, F. (2014). "Redox Flow Batteries for the storage of renewable energy: a review". Renewable & Sustainable Energy Reviews. 29: 325–335. doi:10.1016/j.rser.2013.08.001.
- P. A. Pissoort, in FR Patent 754065 (1933)
- A. Pelligri and P. M. Spaziante, in GB Patent 2030349 (1978), to Oronzio de Nori Impianti Elettrochimici S.p.A.
- M. Rychcik and M. Skyllas-Kazacos, J. Power Sources, 22 (1988) 59–67
- UniEnergy Technologies Products Accessed 21 Jan 2016.
- "Vanadium Redox Flow Batteries" (PDF). Pacific Northwest National Laboratory. October 2012.
- Miller, Kelsey. UniEnergy Technologies Goes from Molecules to Megawatts Archived 31 January 2016 at the Wayback Machine, Clean Tech Alliance, 7 July 2014. Accessed 21 Jan 2016.
- Spagnuolo, G.; Petrone, G.; Mattavelli, P.; Guarnieri, M. (2016). "Vanadium Redox Flow Batteries: Potentials and Challenges of an Emerging Storage Technology". IEEE Industrial Electronics Magazine. 10 (4): 20–31. doi:10.1109/MIE.2016.2611760. hdl:11577/3217695.
- Mustafa, Ibrahim; Lopez, Ivan; Younes, Hammad; Susantyoko, Rahmat Agung; Al-Rub, Rashid Abu; Almheiri, Saif (March 2017). "Fabrication of Freestanding Sheets of Multiwalled Carbon Nanotubes (Buckypapers) for Vanadium Redox Flow Batteries and Effects of Fabrication Variables on Electrochemical Performance". Electrochimica Acta. 230: 222–235. doi:10.1016/j.electacta.2017.01.186. ISSN 0013-4686.
- Mustafa, Ibrahim; Bamgbopa, Musbaudeen O.; Alraeesi, Eman; Shao-Horn, Yang; Sun, Hong; Almheiri, Saif (1 January 2017). "Insights on the Electrochemical Activity of Porous Carbonaceous Electrodes in Non-Aqueous Vanadium Redox Flow Batteries". Journal of the Electrochemical Society. 164 (14): A3673–A3683. doi:10.1149/2.0621714jes. ISSN 0013-4651.
- Mustafa, Ibrahim; Al Shehhi, Asma; Al Hammadi, Ayoob; Susantyoko, Rahmat; Palmisano, Giovanni; Almheiri, Saif (May 2018). "Effects of carbonaceous impurities on the electrochemical activity of multiwalled carbon nanotube electrodes for vanadium redox flow batteries". Carbon. 131: 47–59. doi:10.1016/j.carbon.2018.01.069. ISSN 0008-6223.
- DOE/Pacific Northwest National Laboratory (17 March 2011). "Electric Grid Reliability: Increasing Energy Storage in Vanadium Redox Batteries by 70 Percent". Science Daily. Retrieved 2 March 2015.
- VRB Power Systems FAQ Archived 13 February 2010 at the Wayback Machine
- History of Vanadium Redox Battery
- Sankarasubramanian, Shrihari; Zhang, Yunzhu; Ramani, Vijay (2019). "Methanesulfonic acid-based electrode-decoupled vanadium–cerium redox flow battery exhibits significantly improved capacity and cycle life". Sustainable Energy & Fuels. 3 (9): 2417–2425. doi:10.1039/C9SE00286C. ISSN 2398-4902.
- Allbright, Greg, et. al. A Comparison of Lead Acid to Lithium-ion in Stationary Storage Applications All Cell, March 2012
- Stone, Mike (3 February 2016). "A Look at the Biggest Energy Storage Projects Built Around the World in the Last Year". Retrieved 12 August 2017.
- "DOE Global Energy Storage Database". www.energystorageexchange.org. Archived from the original on 9 November 2017. Retrieved 9 November 2017.
- "Redox-Flow-Batterien". Retrieved 27 July 2014.
- "Der Rotor steht noch still".
- "Großprojekt »RedoxWind«". Fraunhofer-Institut für Chemische Technologie.
- "Energy Storage in China". www.ees-magazine.com. Retrieved 12 August 2017.
- Zonghao, L. I. U.; Huamin, Zhang; Sujun, G. a. O.; Xiangkun, M. A.; Yufeng, L. I. U.; 刘宗浩, 张华民. "The world's largest all-vanadium redox flow battery energy storage system for a wind farm, 风场配套用全球最大全钒液流电池储能系统". 储能科学与技术. 3 (1): 71–77. doi:10.3969/j.issn.2095-4239.2014.01.010.
- "DOE Global Energy Storage Database". www.energystorageexchange.org. Retrieved 9 November 2017.
- "DOE Global Energy Storage Database". www.energystorageexchange.org. Archived from the original on 31 August 2018. Retrieved 9 November 2017.
- "UET and Snohomish County PUD Dedicate the World's Largest Capacity Containerized Flow Battery". Energy Storage News. 29 March 2017. Archived from the original on 18 August 2018. Retrieved 29 December 2017.
- "PUD invests $11.2 million in energy-storing units". Everett Herald. 2 November 2016. Retrieved 29 December 2017.
- "SDG&E and Sumitomo unveil largest vanadium redox flow battery in the US". Energy Storage News. 17 March 2017. Retrieved 12 August 2017.
- Wesoff, Eric, St. John, Jeff. Largest Capacity Flow Battery in North America and EU is Online, Greentech Media, June 2015. Accessed 21 Jan 2016.
- "It's Big and Long-Lived, and It Won't Catch Fire: The Vanadium Redox-Flow Battery". IEEE Spectrum: Technology, Engineering, and Science News. Retrieved 12 November 2017.
- "First phase of China's biggest flow battery put into operation by VRB Energy". Energy Storage News. Retrieved 4 May 2019.
- Steve Wilhelm (3 July 2014). "Liquid battery the size of a truck, will give utilities a charge". Puget Sound Business Journal. Retrieved 2 May 2015.
- Entrepreneur, Office of the Queensland Chief (3 February 2021). "How Queensland can supercharge the future of batteries". Office of the Queensland Chief Entrepreneur. Retrieved 3 February 2021.
- "StorEn Tech Provides First Of Its Kind Vanadium Flow Battery To Australia". CleanTechnica. 19 December 2020. Retrieved 3 February 2021.
- BILL HAGSTRAND (23 August 2013). "Vanadium redox: powering up local communities". Crain's Cleveland Business. Retrieved 2 May 2015.
- "US clean-tech investments leap to US$1.1bn. Where's Ireland at?". Silicon Republic. 11 April 2011. Retrieved 2 May 2015.
- "Voltstorage develops a safe and ecological storage solution". 16 January 2018.
- Jeff St. John (2 March 2010). "Made in China: Prudent Energy Lands $22M For Flow Batteries". GigaOm. Retrieved 2 May 2015.
- "Australian Vanadium Ltd ships first vanadium flow battery from Austria". Proactive Investors. 13 July 2016. Retrieved 24 November 2017.
Additional references
- Presentation paper from the IEEE summer 2001 conference
- UNSW Site on Vanadium batteries
- Report by World Energy
- World Map Of Global Vanadium Deposits Vanadium geology is fairly unusual compared to a base metals ore body.
- "Improved Redox Flow Batteries For Electric Cars". ScienceDaily/Fraunhofer-Gesellschaft. 13 October 2009. Retrieved 21 June 2014.
External links
- VRFB developments at UNSW
- VRB at everything2
- The Need for Vanadium Redox Energy Storage in Wind Turbine Generators Net electricity generation from all forms of renewable energies in America increased by over 15% between 2005 and 2009.
- redT and Avalon have merged as Invinity Energy Systems, a global leader in Vanadium Flow Batteries