Subacute sclerosing panencephalitis
Subacute sclerosing panencephalitis (SSPE)—also known as Dawson disease—is a rare form of chronic progressive brain inflammation caused by slow infection with certain defective strains of hypermutated measles virus. The condition primarily affects children, teens, and young adults. It has been estimated that about 1 in 10,000 people who get measles will eventually develop SSPE.[1] However, a 2016 study estimated that the rate for babies who contracted measles was as high as 1 in 609.[2] No cure for SSPE exists, and the condition is almost always fatal. SSPE should not be confused with acute disseminated encephalomyelitis, which can also be caused by the measles virus, but has a very different timing and course.[3]
| Subacute sclerosing panencephalitis | |
|---|---|
| Other names | Dawson disease |
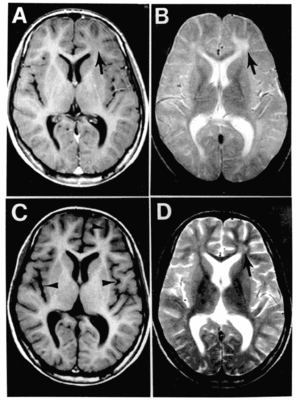 | |
| Subacute sclerosing panencephalitis. | |
| Specialty | Infectious disease |
| Symptoms | Behavior changes, seizures, spasticity, poor coordination, coma |
| Usual onset | 6-15 years after infection with measles |
| Causes | Measles virus |
| Risk factors | Measles infection |
| Diagnostic method | EEG, Serologic testing, brain biopsy |
| Prevention | Measles vaccine |
| Treatment | Supportive treatment |
| Medication | Intrathecal interferon alpha, intravenous ribavirin |
| Prognosis | Usually fatal |
| Frequency | About 1 in 10,000 people infected by measles[1] |
SSPE is caused by the wild-type virus, not by vaccine strains.[4][5]
Signs and symptoms
SSPE is characterized by a history of primary measles infection, followed by an asymptomatic period that lasts 7 years on average but can range from 1 month to 27 years. After the asymptomatic period, progressive neurological deterioration occurs, characterized by behavior change, intellectual problems, myoclonic seizures, blindness, ataxia, and eventually death.[6][7]
Progression
Symptoms progress through the following 4 stages:[8]
- Stage 1: There may be personality changes, mood swings, or depression. Fever and headache may also be present. This stage may last up to 6 months.
- Stage 2: This stage may involve jerking, muscle spasms, seizures, loss of vision, and dementia.
- Stage 3: Jerking movements are replaced by writhing (twisting) movements and rigidity. At this stage complications may result in death.
- Stage 4: The final stage, in which breathing, heart rate, and blood pressure are affected, leading to coma and death.
Pathogenesis
A large number of nucleocapsids are produced in the neurons and the glial cells. In these cells the viral genes that encode envelope proteins have restricted expression.[9] As a result, infectious particles like the M protein are not produced, and the virus is able to survive persistently without evoking an immune response. Eventually the infection will lead to SSPE.[10]
Diagnosis
According to the Merck Manual:[7]
"SSPE is suspected in young patients with dementia and neuromuscular irritability. EEG, CT or MRI, CSF examination, and measles serologic testing are done. EEG shows periodic complexes with high-voltage diphasic waves occurring synchronously throughout the recording. CT or MRI may show cortical atrophy or white matter lesions. CSF examination usually reveals normal pressure, cell count, and total protein content; however, CSF globulin is almost always elevated, constituting up to 20 to 60% of CSF protein. Serum and CSF contain elevated levels of measles virus antibodies. Anti-measles IgG appears to increase as the disease progresses. If test results are inconclusive, brain biopsy may be needed."
Treatment
If the diagnosis is made during stage 1 it may be possible to treat the disease with oral isoprinosine (Inosiplex) and intraventricular interferon alfa, but the response to these drugs varies from patient to patient,[11] and the only accepted treatments are supportive measures such as anticonvulsants.[7]
Prognosis
In the classic presentation of the disease death occurs in 1 to 3 years,[12] but faster and slower progressions can occur. Faster deterioration in cases of acute fulminant SSPE leads to death within 3 months of diagnosis.[13][14]Although the prognosis is bleak for SSPE past stage 1, there is a 5% spontaneous remission rate—this may be either a full remission that may last many years or an improvement in condition giving a longer progression period or at least a longer period with the less severe symptoms.[14][15]
Epidemiology
SSPE is a rare condition, although there is still relatively high incidence in Asia and the Middle East. However, the number of reported cases is declining since the introduction of the measles vaccine—eradication of the measles virus prevents the SSPE mutation and therefore the progression of the disease or even the initial infection itself.
References
- Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, Zaki SR, Shieh WJ, Rota PA (2005). "Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized". The Journal of Infectious Diseases. 192 (10): 1686–1693. doi:10.1086/497169. PMID 16235165.
- Sun, Lena (October 28, 2016). "New data shows a deadly measles complication is more common than thought". The Washington Post. Retrieved October 28, 2016.
- Fisher DL, Defres S, Solomon T (2015). "Measles-induced encephalitis". QJM. 108 (3): 177–182. doi:10.1093/qjmed/hcu113. PMID 24865261.CS1 maint: uses authors parameter (link)
- Jafri, Sidra K; Kumar, Raman; Ibrahim, Shahnaz H (2018-06-26). "Subacute sclerosing panencephalitis – current perspectives". Pediatric Health, Medicine and Therapeutics. 9: 67–71. doi:10.2147/PHMT.S126293. ISSN 1179-9927. PMC 6027681. PMID 29985487.
- Campbell, H; Andrews, N; Brown, K E; Miller, E (2007). "Review of the effect of measles vaccination on the epidemiology of SSPE". International Journal of Epidemiology. 36 (6): 1334–1348. doi:10.1093/ije/dym207. PMID 18037676.
- "CDC pinkbook". 2019-03-29.
- "merckmanuals.com".
- "medline.gov".
- Jawetz. Melnick & Adelberg's Medical Microbiology. Lange. 2010. p. 586. ISBN 978-0-07-174271-9.
- Carter, M. J.; Willcocks, M. M.; Ter Meulen, V. (1983). "Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line". Nature. 305 (5930): 153–5. Bibcode:1983Natur.305..153C. doi:10.1038/305153a0. PMC 7094927. PMID 6888557.
-
- Gascon G, Yamanis S, Crowell J, et al. Combined oralisoprinosine-intraventricular alpha-interferon therapy for subacute sclerosing panencephalitis. Brain Dev. 1993; 15:346–55.
- Anlar B, Yalaz K, Oktem F; et al. (1997). "Long-term follow-up of patients with subacute sclerosing panencephalitis treated with intraventricular alpha-interferon". Neurology. 48 (2): 526–8. doi:10.1212/wnl.48.2.526. PMID 9040751. S2CID 20412574.CS1 maint: multiple names: authors list (link)
- Cianchetti C, Marrosu MG, Muntoni F; et al. (1998). "Intraventricularalpha-interferon in subacute sclerosing panencephalitis". Neurology. 50 (1): 315–16. doi:10.1212/wnl.50.1.315. PMID 9443512. S2CID 33700234.CS1 maint: multiple names: authors list (link)
- "SSPE information page www.ninds.nih.gov".
- Risk WS, Haddad FS (1979). "The variable natural history of subacute sclerosing panencephalitis: a study of 118 cases from the Middle East". Arch Neurol. 56 (10): 610–14. doi:10.1001/archneur.1979.00500460044004. PMID 485888.
- Garg, R K (1 February 2002). "Subacute sclerosing panencephalitis". Postgraduate Medical Journal. 78 (916): 63–70. doi:10.1136/pmj.78.916.63. PMC 1742261. PMID 11807185.
-
- Grunewald T, Lampe J, Weissbrich B; et al. (1998). "A 35-year old bricklayer with hemimyoclonic jerks". Lancet. 351 (9120): 1926. doi:10.1016/s0140-6736(98)04091-4. PMID 9654261. S2CID 206010725.CS1 maint: multiple names: authors list (link)
- Santoshkumar B, Radhakrishnan K (1998). "Substantial spontaneous long-term remission in subacute sclerosing panencephalitis (SSPE)". J Neurol Sci. 154 (1): 83–8. doi:10.1016/s0022-510x(97)00303-1. PMID 9543327. S2CID 10635605.
Further reading
- Bonthius D, Stanek N, Grose C (2000). "Subacute sclerosing panencephalitis, a measles complication, in an internationally adopted child". Emerg Infect Dis. 6 (4): 377–81. doi:10.3201/eid0604.000409. PMC 2640885. PMID 10905971.
External links
- 23-273l. at Merck Manual of Diagnosis and Therapy Home Edition
- subacute_panencephalitis at NINDS
- PubMed Health: Subacute sclerosing panencephalitis
- synd/1889 at Who Named It?
| Classification | |
|---|---|
| External resources |