Hepatitis B
Hepatitis B is an infectious disease caused by the hepatitis B virus (HBV) that affects the liver;[1][7] it is a type of viral hepatitis.[8] It can cause both acute and chronic infection.[1] Many people have no symptoms during the initial infection.[1] In acute infection, some may develop a rapid onset of sickness with vomiting, yellowish skin, tiredness, dark urine, and abdominal pain.[1] Often these symptoms last a few weeks and rarely does the initial infection result in death.[1][9] It may take 30 to 180 days for symptoms to begin.[1] In those who get infected around the time of birth 90% develop chronic hepatitis B while less than 10% of those infected after the age of five do.[4] Most of those with chronic disease have no symptoms; however, cirrhosis and liver cancer may eventually develop.[2] Cirrhosis or liver cancer occur in about 25% of those with chronic disease.[1]
| Hepatitis B | |
|---|---|
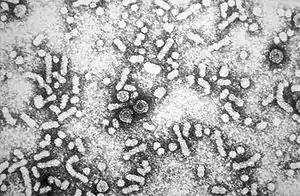 | |
| Electron micrograph of hepatitis B virus | |
| Specialty | Infectious disease, gastroenterology |
| Symptoms | None, yellowish skin, tiredness, dark urine, abdominal pain[1] |
| Complications | Cirrhosis, liver cancer[2] |
| Usual onset | Symptoms may take up to 6 months to appear[1] |
| Duration | Short or long term[3] |
| Causes | Hepatitis B virus spread by some body fluids[1] |
| Risk factors | Intravenous drug use, sexual intercourse, dialysis, living with an infected person[1][4] |
| Diagnostic method | Blood tests[1] |
| Prevention | Hepatitis B vaccine[1] |
| Treatment | Antiviral medication (tenofovir, interferon), liver transplantation[1] |
| Frequency | >391 million (2017)[5] |
| Deaths | 65,400 direct (2015), >750,000 (total)[1][6] |
The virus is transmitted by exposure to infectious blood or body fluids.[1] Infection around the time of birth or from contact with other people's blood during childhood is the most frequent method by which hepatitis B is acquired in areas where the disease is common.[1] In areas where the disease is rare, intravenous drug use and sexual intercourse are the most frequent routes of infection.[1] Other risk factors include working in healthcare, blood transfusions, dialysis, living with an infected person, travel in countries where the infection rate is high, and living in an institution.[1][4] Tattooing and acupuncture led to a significant number of cases in the 1980s; however, this has become less common with improved sterilization.[10] The hepatitis B viruses cannot be spread by holding hands, sharing eating utensils, kissing, hugging, coughing, sneezing, or breastfeeding.[4] The infection can be diagnosed 30 to 60 days after exposure.[1] The diagnosis is usually confirmed by testing the blood for parts of the virus and for antibodies against the virus.[1] It is one of five main hepatitis viruses: A, B, C, D, and E.[11]
The infection has been preventable by vaccination since 1982.[1][12] Vaccination is recommended by the World Health Organization in the first day of life if possible.[1] Two or three more doses are required at a later time for full effect.[1] This vaccine works about 95% of the time.[1] About 180 countries gave the vaccine as part of national programs as of 2006.[13] It is also recommended that all blood be tested for hepatitis B before transfusion, and that condoms be used to prevent infection.[1] During an initial infection, care is based on the symptoms that a person has.[1] In those who develop chronic disease, antiviral medication such as tenofovir or interferon may be useful; however, these drugs are expensive.[1] Liver transplantation is sometimes used for cirrhosis.[1]
About a third of the world population has been infected at one point in their lives.[1] At least 391 million people, or 5% of the world's population, had chronic HBV infection as of 2017.[5] While another 145 million cases of acute HBV infection occurred that year.[5] Over 750,000 people die of hepatitis B each year.[1] About 300,000 of these are due to liver cancer.[14] The disease is most common in the Western Pacific (6.2%) and African (6.1%) regions.[11] In Europe rates are 1.6% and in the Americas they are 0.7%.[1] It was originally known as "serum hepatitis".[15]
Signs and symptoms
Acute infection with hepatitis B virus is associated with acute viral hepatitis, an illness that begins with general ill-health, loss of appetite, nausea, vomiting, body aches, mild fever, and dark urine, and then progresses to development of jaundice. The illness lasts for a few weeks and then gradually improves in most affected people. A few people may have a more severe form of liver disease known as fulminant hepatic failure and may die as a result. The infection may be entirely asymptomatic and may go unrecognized.[16]
Chronic infection with hepatitis B virus either may be asymptomatic or may be associated with a chronic inflammation of the liver (chronic hepatitis), leading to cirrhosis over a period of several years. This type of infection dramatically increases the incidence of hepatocellular carcinoma (HCC; liver cancer). Across Europe, hepatitis B and C cause approximately 50% of hepatocellular carcinomas.[17][18] Chronic carriers are encouraged to avoid consuming alcohol as it increases their risk for cirrhosis and liver cancer. Hepatitis B virus has been linked to the development of membranous glomerulonephritis (MGN).[19]
Symptoms outside of the liver are present in 1–10% of HBV-infected people and include serum-sickness–like syndrome, acute necrotizing vasculitis (polyarteritis nodosa), membranous glomerulonephritis, and papular acrodermatitis of childhood (Gianotti–Crosti syndrome).[20][21] The serum-sickness–like syndrome occurs in the setting of acute hepatitis B, often preceding the onset of jaundice.[22] The clinical features are fever, skin rash, and polyarteritis. The symptoms often subside shortly after the onset of jaundice but can persist throughout the duration of acute hepatitis B.[23] About 30–50% of people with acute necrotizing vasculitis (polyarteritis nodosa) are HBV carriers.[24] HBV-associated nephropathy has been described in adults but is more common in children.[25][26] Membranous glomerulonephritis is the most common form.[23] Other immune-mediated hematological disorders, such as essential mixed cryoglobulinemia and aplastic anemia have been described as part of the extrahepatic manifestations of HBV infection, but their association is not as well-defined; therefore, they probably should not be considered etiologically linked to HBV.[23]
Cause
Transmission
Transmission of hepatitis B virus results from exposure to infectious blood or body fluids containing blood. It is 50 to 100 times more infectious than human immunodeficiency virus (HIV).[27] Possible forms of transmission include sexual contact,[28] blood transfusions and transfusion with other human blood products,[29] re-use of contaminated needles and syringes,[30] and vertical transmission from mother to child (MTCT) during childbirth. Without intervention, a mother who is positive for HBsAg has a 20% risk of passing the infection to her offspring at the time of birth. This risk is as high as 90% if the mother is also positive for HBeAg. HBV can be transmitted between family members within households, possibly by contact of nonintact skin or mucous membrane with secretions or saliva containing HBV.[31] However, at least 30% of reported hepatitis B among adults cannot be associated with an identifiable risk factor.[32] Breastfeeding after proper immunoprophylaxis does not appear to contribute to mother-to-child-transmission (MTCT) of HBV.[33] The virus may be detected within 30 to 60 days after infection and can persist and develop into chronic hepatitis B. The incubation period of the hepatitis B virus is 75 days on average but can vary from 30 to 180 days.[34]
Virology
Structure
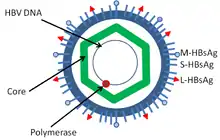
Hepatitis B virus (HBV) is a member of the hepadnavirus family.[35] The virus particle (virion) consists of an outer lipid envelope and an icosahedral nucleocapsid core composed of core protein. These virions are 30–42 nm in diameter. The nucleocapsid encloses the viral DNA and a DNA polymerase that has reverse transcriptase activity.[36] The outer envelope contains embedded proteins that are involved in viral binding of, and entry into, susceptible cells. The virus is one of the smallest enveloped animal viruses. The 42 nm virions, which are capable of infecting liver cells known as hepatocytes, are referred to as "Dane particles".[37] In addition to the Dane particles, filamentous and spherical bodies lacking a core can be found in the serum of infected individuals. These particles are not infectious and are composed of the lipid and protein that forms part of the surface of the virion, which is called the surface antigens (HBsAg), and is produced in excess during the life cycle of the virus.[38]
Genome
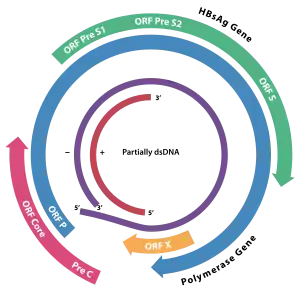
The genome of HBV is made of circular DNA, but it is unusual because the DNA is not fully double-stranded. One end of the full length strand is linked to the viral DNA polymerase. The genome is 3020–3320 nucleotides long (for the full-length strand) and 1700–2800 nucleotides long (for the short length-strand).[39] The negative-sense (non-coding) is complementary to the viral mRNA. The viral DNA is found in the nucleus soon after infection of the cell. The partially double-stranded DNA is rendered fully double-stranded by completion of the (+) sense strand and removal of a protein molecule from the (−) sense strand and a short sequence of RNA from the (+) sense strand. Non-coding bases are removed from the ends of the (−) sense strand and the ends are rejoined. There are four known genes encoded by the genome, called C, X, P, and S. The core protein is coded for by gene C (HBcAg), and its start codon is preceded by an upstream in-frame AUG start codon from which the pre-core protein is produced. HBeAg is produced by proteolytic processing of the pre-core protein. In some rare strains of the virus known as Hepatitis B virus precore mutants, no HBeAg is present.[40] The DNA polymerase is encoded by gene P. Gene S is the gene that codes for the surface antigen (HBsAg). The HBsAg gene is one long open reading frame but contains three in frame "start" (ATG) codons that divide the gene into three sections, pre-S1, pre-S2, and S. Because of the multiple start codons, polypeptides of three different sizes called large (the order from surface to the inside: pre-S1, pre-S2, and S ), middle (pre-S2, S), and small (S)[41] are produced.[42] There is a myristyl group, which plays an important role in infection, on the amino-terminal end of the preS1 part of the large (L) protein.[43] In addition to that, N terminus of the L protein have virus attachment and capsid binding sites. Because of that, the N termini of half of the L protein molecules are positioned outside the membrane and the other half positioned inside the membrane.[44]
The function of the protein coded for by gene X is not fully understood but it is associated with the development of liver cancer. It stimulates genes that promote cell growth and inactivates growth regulating molecules.[45]
Pathogenesis
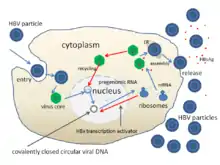
The life cycle of hepatitis B virus is complex. Hepatitis B is one of a few known pararetroviruses: non-retroviruses that still use reverse transcription in their replication process. The virus gains entry into the cell by binding to NTCP[46] on the surface and being endocytosed. Because the virus multiplies via RNA made by a host enzyme, the viral genomic DNA has to be transferred to the cell nucleus by host proteins called chaperones. The partially double-stranded viral DNA is then made fully double stranded by a viral polymerase and transformed into covalently closed circular DNA (cccDNA). This cccDNA serves as a template for transcription of four viral mRNAs by host RNA polymerase. The largest mRNA, (which is longer than the viral genome), is used to make the new copies of the genome and to make the capsid core protein and the viral DNA polymerase. These four viral transcripts undergo additional processing and go on to form progeny virions that are released from the cell or returned to the nucleus and re-cycled to produce even more copies.[42][47] The long mRNA is then transported back to the cytoplasm where the virion P protein (the DNA polymerase) synthesizes DNA via its reverse transcriptase activity.
Serotypes and genotypes
The virus is divided into four major serotypes (adr, adw, ayr, ayw) based on antigenic epitopes presented on its envelope proteins, and into eight major genotypes (A–H). The genotypes have a distinct geographical distribution and are used in tracing the evolution and transmission of the virus. Differences between genotypes affect the disease severity, course and likelihood of complications, and response to treatment and possibly vaccination.[48][49] There are two other genotypes I and J but they are not universally accepted as of 2015.[50] The diversity of genotypes is not shown equally in the world. For example, A, D, and E genotypes have been seen in Africa prevalently while B and C genotypes are observed in Asia as widespread.[51]
Genotypes differ by at least 8% of their sequence and were first reported in 1988 when six were initially described (A–F).[52] Two further types have since been described (G and H).[53] Most genotypes are now divided into subgenotypes with distinct properties.[54]
Mechanisms
Hepatitis B virus primarily interferes with the functions of the liver by replicating in hepatocytes. A functional receptor is NTCP.[46] There is evidence that the receptor in the closely related duck hepatitis B virus is carboxypeptidase D.[55][56] The virions bind to the host cell via the preS domain of the viral surface antigen and are subsequently internalized by endocytosis. HBV-preS-specific receptors are expressed primarily on hepatocytes; however, viral DNA and proteins have also been detected in extrahepatic sites, suggesting that cellular receptors for HBV may also exist on extrahepatic cells.[57]
During HBV infection, the host immune response causes both hepatocellular damage and viral clearance. Although the innate immune response does not play a significant role in these processes, the adaptive immune response, in particular virus-specific cytotoxic T lymphocytes(CTLs), contributes to most of the liver injury associated with HBV infection. CTLs eliminate HBV infection by killing infected cells and producing antiviral cytokines, which are then used to purge HBV from viable hepatocytes.[58] Although liver damage is initiated and mediated by the CTLs, antigen-nonspecific inflammatory cells can worsen CTL-induced immunopathology, and platelets activated at the site of infection may facilitate the accumulation of CTLs in the liver.[59]
Diagnosis
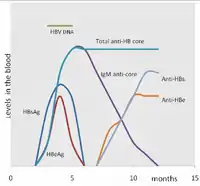
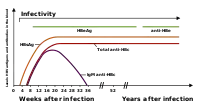
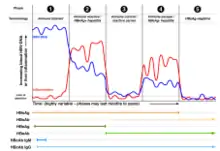
The tests, called assays, for detection of hepatitis B virus infection involve serum or blood tests that detect either viral antigens (proteins produced by the virus) or antibodies produced by the host. Interpretation of these assays is complex.[60]
The hepatitis B surface antigen (HBsAg) is most frequently used to screen for the presence of this infection. It is the first detectable viral antigen to appear during infection. However, early in an infection, this antigen may not be present and it may be undetectable later in the infection as it is being cleared by the host. The infectious virion contains an inner "core particle" enclosing viral genome. The icosahedral core particle is made of 180 or 240 copies of the core protein, alternatively known as hepatitis B core antigen, or HBcAg. During this 'window' in which the host remains infected but is successfully clearing the virus, IgM antibodies specific to the hepatitis B core antigen (anti-HBc IgM) may be the only serological evidence of disease. Therefore, most hepatitis B diagnostic panels contain HBsAg and total anti-HBc (both IgM and IgG).[61]
Shortly after the appearance of the HBsAg, another antigen called hepatitis B e antigen (HBeAg) will appear. Traditionally, the presence of HBeAg in a host's serum is associated with much higher rates of viral replication and enhanced infectivity; however, variants of the hepatitis B virus do not produce the 'e' antigen, so this rule does not always hold true.[62] During the natural course of an infection, the HBeAg may be cleared, and antibodies to the 'e' antigen (anti-HBe) will arise immediately afterwards. This conversion is usually associated with a dramatic decline in viral replication.
If the host is able to clear the infection, eventually the HBsAg will become undetectable and will be followed by IgG antibodies to the hepatitis B surface antigen and core antigen (anti-HBs and anti HBc IgG).[35] The time between the removal of the HBsAg and the appearance of anti-HBs is called the window period. A person negative for HBsAg but positive for anti-HBs either has cleared an infection or has been vaccinated previously.
Individuals who remain HBsAg positive for at least six months are considered to be hepatitis B carriers.[63] Carriers of the virus may have chronic hepatitis B, which would be reflected by elevated serum alanine aminotransferase (ALT) levels and inflammation of the liver, if they are in the immune clearance phase of chronic infection. Carriers who have seroconverted to HBeAg negative status, in particular those who acquired the infection as adults, have very little viral multiplication and hence may be at little risk of long-term complications or of transmitting infection to others.[64] However, it is possible for individuals to enter an "immune escape" with HBeAg-negative hepatitis.
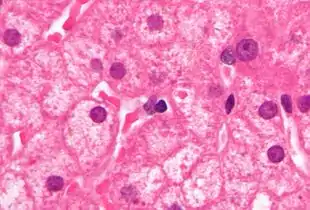
PCR tests have been developed to detect and measure the amount of HBV DNA, called the viral load, in clinical specimens. These tests are used to assess a person's infection status and to monitor treatment.[65] Individuals with high viral loads, characteristically have ground glass hepatocytes on biopsy.
Prevention
Vaccine
Vaccines for the prevention of hepatitis B have been routinely recommended for babies since 1991 in the United States.[66] The first dose is generally recommended within a day of birth.[67] The hepatitis B vaccine was the first vaccine capable of preventing cancer, specifically liver cancer.[68]
Most vaccines are given in three doses over a course of months. A protective response to the vaccine is defined as an anti-HBs antibody concentration of at least 10 mIU/ml in the recipient's serum. The vaccine is more effective in children and 95 percent of those vaccinated have protective levels of antibody. This drops to around 90% at 40 years of age and to around 75 percent in those over 60 years. The protection afforded by vaccination is long lasting even after antibody levels fall below 10 mIU/ml. For newborns of HBsAg-positive mothers: hepatitis B vaccine alone, hepatitis B immunoglobulin alone, or the combination of vaccine plus hepatitis B immunoglobulin, all prevent hepatitis B occurrence.[69] Furthermore, the combination of vaccine plus hepatitis B immunoglobulin is superior to vaccine alone.[69] This combination prevents HBV transmission around the time of birth in 86% to 99% of cases.[70]
Tenofovir given in the second or third trimester can reduce the risk of mother to child transmission by 77% when combined with hepatitis B immunoglobulin and the hepatitis B vaccine, especially for pregnant women with high hepatitis B virus DNA levels.[71] However, there is no sufficient evidence that the administration of hepatitis B immunoglobulin alone during pregnancy, might reduce transmission rates to the newborn infant.[72] No randomized control trial has been conducted to assess the effects of hepatitis B vaccine during pregnancy for preventing infant infection.[73]
All those with a risk of exposure to body fluids such as blood should be vaccinated, if not already.[66] Testing to verify effective immunization is recommended and further doses of vaccine are given to those who are not sufficiently immunized.[66]
In 10- to 22-year follow-up studies there were no cases of hepatitis B among those with a normal immune system who were vaccinated. Only rare chronic infections have been documented.[74] Vaccination is particularly recommended for high risk groups including: health workers, people with chronic kidney failure, and men who have sex with men.[75][76][77]
Both types of the hepatitis B vaccine, the plasma-derived vaccine (PDV) and recombinant vaccine (RV) are of similar effectiveness in preventing the infection in both healthcare workers and chronic kidney failure groups.[75][76] With one difference noticed among health worker group, that the RV intramuscular route is significantly more effective compared with RV intradermal route of administration.[75]
Other
In assisted reproductive technology, sperm washing is not necessary for males with hepatitis B to prevent transmission, unless the female partner has not been effectively vaccinated.[78] In females with hepatitis B, the risk of transmission from mother to child with IVF is no different from the risk in spontaneous conception.[78]
Those at high risk of infection should be tested as there is effective treatment for those who have the disease.[79] Groups that screening is recommended for include those who have not been vaccinated and one of the following: people from areas of the world where hepatitis B occurs in more than 2%, those with HIV, intravenous drug users, men who have sex with men, and those who live with someone with hepatitis B.[79] Screening during pregnancy is recommended in the United States.[80]
Treatment
Acute hepatitis B infection does not usually require treatment and most adults clear the infection spontaneously.[81][82] Early antiviral treatment may be required in fewer than 1% of people, whose infection takes a very aggressive course (fulminant hepatitis) or who are immunocompromised. On the other hand, treatment of chronic infection may be necessary to reduce the risk of cirrhosis and liver cancer. Chronically infected individuals with persistently elevated serum alanine aminotransferase, a marker of liver damage, and HBV DNA levels are candidates for therapy.[83] Treatment lasts from six months to a year, depending on medication and genotype.[84] Treatment duration when medication is taken by mouth, however, is more variable and usually longer than one year.[85]
Although none of the available medications can clear the infection, they can stop the virus from replicating, thus minimizing liver damage. As of 2018, there are eight medications licensed for the treatment of hepatitis B infection in the United States. These include antiviral medications lamivudine, adefovir, tenofovir disoproxil, tenofovir alafenamide, telbivudine, and entecavir, and the two immune system modulators interferon alpha-2a and PEGylated interferon alpha-2a. In 2015 the World Health Organization recommended tenofovir or entecavir as first-line agents.[86] Those with current cirrhosis are in most need of treatment.[86]
The use of interferon, which requires injections daily or thrice weekly, has been supplanted by long-acting PEGylated interferon, which is injected only once weekly.[87] However, some individuals are much more likely to respond than others, and this might be because of the genotype of the infecting virus or the person's heredity. The treatment reduces viral replication in the liver, thereby reducing the viral load (the amount of virus particles as measured in the blood).[88] Response to treatment differs between the genotypes. Interferon treatment may produce an e antigen seroconversion rate of 37% in genotype A but only a 6% seroconversion in type D. Genotype B has similar seroconversion rates to type A while type C seroconverts only in 15% of cases. Sustained e antigen loss after treatment is ~45% in types A and B but only 25–30% in types C and D.[89]
Prognosis
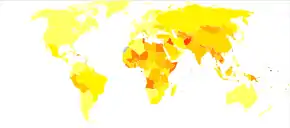
|
no data
<10
10–20
20–40
40–60
60–80
80–100
|
100–125
125–150
150–200
200–250
250–500
>500
|
Hepatitis B virus infection may be either acute (self-limiting) or chronic (long-standing). Persons with self-limiting infection clear the infection spontaneously within weeks to months.
Children are less likely than adults to clear the infection. More than 95% of people who become infected as adults or older children will stage a full recovery and develop protective immunity to the virus. However, this drops to 30% for younger children, and only 5% of newborns that acquire the infection from their mother at birth will clear the infection.[90] This population has a 40% lifetime risk of death from cirrhosis or hepatocellular carcinoma.[87] Of those infected between the age of one to six, 70% will clear the infection.[91]
Hepatitis D (HDV) can occur only with a concomitant hepatitis B infection, because HDV uses the HBV surface antigen to form a capsid.[92] Co-infection with hepatitis D increases the risk of liver cirrhosis and liver cancer.[93] Polyarteritis nodosa is more common in people with hepatitis B infection.
Cirrhosis
A number of different tests are available to determine the degree of cirrhosis present. Transient elastography (FibroScan) is the test of choice, but it is expensive.[86] Aspartate aminotransferase to platelet ratio index may be used when cost is an issue.[86]
Reactivation
Hepatitis B virus DNA remains in the body after infection, and in some people, including those that do not have detectable HBsAg, the disease recurs.[94][95] Although rare, reactivation is seen most often following alcohol or drug use,[96] or in people with impaired immunity.[97] HBV goes through cycles of replication and non-replication. Approximately 50% of overt carriers experience acute reactivation. Males with baseline ALT of 200 UL/L are three times more likely to develop a reactivation than people with lower levels. Although reactivation can occur spontaneously,[98] people who undergo chemotherapy have a higher risk.[99] Immunosuppressive drugs favor increased HBV replication while inhibiting cytotoxic T cell function in the liver.[100] The risk of reactivation varies depending on the serological profile; those with detectable HBsAg in their blood are at the greatest risk, but those with only antibodies to the core antigen are also at risk. The presence of antibodies to the surface antigen, which are considered to be a marker of immunity, does not preclude reactivation.[99] Treatment with prophylactic antiviral drugs can prevent the serious morbidity associated with HBV disease reactivation.[99]
Epidemiology
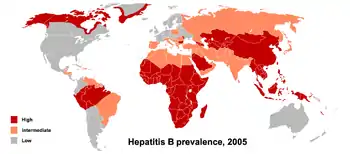

At least 391 million people, or 5% of the world's population, had chronic HBV infection as of 2017.[5] While another 145 million cases of acute HBV infection occurred that year.[5] Regional prevalences range from around 6% in Africa to 0.7% in the Americas.[102]
Routes of infection include vertical transmission (such as through childbirth), early life horizontal transmission (bites, lesions, and sanitary habits), and adult horizontal transmission (sexual contact, intravenous drug use).[103]
The primary method of transmission reflects the prevalence of chronic HBV infection in a given area. In low prevalence areas such as the continental United States and Western Europe, injection drug abuse and unprotected sex are the primary methods, although other factors may also be important.[104] In moderate prevalence areas, which include Eastern Europe, Russia, and Japan, where 2–7% of the population is chronically infected, the disease is predominantly spread among children. In high-prevalence areas such as China and South East Asia, transmission during childbirth is most common, although in other areas of high endemicity such as Africa, transmission during childhood is a significant factor.[105] The prevalence of chronic HBV infection in areas of high endemicity is at least 8% with 10–15% prevalence in Africa/Far East.[106] As of 2010, China has 120 million infected people, followed by India and Indonesia with 40 million and 12 million, respectively. According to World Health Organization (WHO), an estimated 600,000 people die every year related to the infection.[107]
In the United States about 19,000 new cases occurred in 2011 down nearly 90% from 1990.[66]
History
The hepatitis B virus has infected humans since at least the Bronze Age.[108][109] The evidence was obtained from 4,500-year-old human remains.[109] According to the 2018 study, the viral genomes obtained by shotgun sequencing became the oldest ever recovered from vertebrate samples.[109] It was also found that some ancient hepatitis viral strains still infect humans, while other became extinct.[109] This disproved the belief that hepatitis B originated in the New World and spread to Europe around 16th century.[109] Another 2018 study of the remains of a mummified child found in the Basilica of San Domenico Maggiore in Naples concluded that the child, who had lived in the 16th century, had a form of HBV, and that the virus was closely related to modern variants.[110] Genomic studies though confirm an older origin in humans. A particular Hepatitis B subgenotype C4 is present in Australian aborigines, and nowhere else in South East Asia, suggesting an ancient origin as much as 50,000 years old.[111][112] Other studies have confirmed that the virus was present in humans 40,000 years ago, and co-spread with them.[113]
The earliest record of an epidemic caused by hepatitis B virus was made by Lurman in 1885.[114] An outbreak of smallpox occurred in Bremen in 1883 and 1,289 shipyard employees were vaccinated with lymph from other people. After several weeks, and up to eight months later, 191 of the vaccinated workers became ill with jaundice and were diagnosed as suffering from serum hepatitis. Other employees who had been inoculated with different batches of lymph remained healthy. Lurman's paper, now regarded as a classical example of an epidemiological study, proved that contaminated lymph was the source of the outbreak. Later, numerous similar outbreaks were reported following the introduction, in 1909, of hypodermic needles that were used, and, more importantly, reused, for administering Salvarsan for the treatment of syphilis.
The largest outbreak of Hepatitis B in history was the infection of 330,000 American soldiers between 1941 and 1942 causing 50,000 to develop jaundice.[115] Not disclosed to the public until 1987, this was traced by the US Public health service to a contaminated yellow-fever vaccine made from human serum of a chronic carrier. The contaminated yellow-fever vaccine had been developed by Eugen Haagen while on a Rockefeller Foundation research fellowship in New York City in 1937.[116] Hagen would go on to become a leading virologist in Nazi Germany. Research on the American epidemic and the Hepatitis B disease model was carried out clandestinely to avoid a scandal and developed into a secret Cold War race with the Soviet Union to fix the Hepatitis B virus contamination problem with the Yellow Fever vaccine. Starting in 1942, Dr. Jonas Salk and Dr. W. Paul Havens, Jr. conducted a number of unethical experiments on prisoners and mental institution patients intentionally exposing them to hepatitis, allowing them to differentiate Hepatitis A from Hepatitis B.[117] Simultaneously, Nazi doctors including Hagen forced concentration camp prisoners (including children) to eat material scraped out of the stomachs of people who had turned yellow from liver disease that they determined did not have Hepatitis A. When the prisoners subsequently sickened with jaundice, the Nazi doctors determined it was a new infectious agent.[118][119]
The virus was not publicly discovered until 1966 when Baruch Blumberg, then working at the National Institutes of Health (NIH), discovered the Australia antigen (later known to be hepatitis B surface antigen, or HBsAg) in the blood of Aboriginal Australian people.[120] Although a virus had been suspected since the research published by Frederick MacCallum in 1947,[121] David Dane and others discovered the virus particle in 1970 by electron microscopy.[122] In 1971, the FDA issued its first-ever blood supply screening order to blood banks.[123] By the early 1980s the genome of the virus had been sequenced.[124]
In 1973, Dr. Wolfgang Szmusness, a Polish/Jewish emigre from the Soviet Union (with a backstory that may be Cold-War propaganda) collaborated with Dr. Saul Krugman of New York University to develop a vaccine. This was first tested for safety by Krugman on mentally retarded children at Willowbrook Hospital in Staten Island, which an investigation later revealed was a frequent participant in questionable medical studies. Dr. Szmuness worked with the Gay Men's Health Project, a scrappy charity in the Meatpacking district of Manhattan, and it had been discovered up to 50% of gay men in the highly promiscuous "gay ghettos" of the post-Stonewall era had been exposed to Hepatitis B.[125] Such "fast-track" homosexuals created the perfect test population for a rigorously-designed trial of the vaccine's effectiveness, and public health policy was adopted to facilitate the vaccine experiment involving 10,000 gay men (creating an environment where sexual transmission among participants was likely), such non-enforcement of existing health codes in establishments such as New York's Mineshaft club, tolerating widespread use of aphrodisiac drugs, and stopping police raids.[126][127] The vaccine trial was also repeated in gay communities in San Francisco, Los Angeles, Denver, St. Louis, and Chicago.[128] The vaccine was approved in 1981, and the trial holds a record for the quickest vaccine to be developed from the identification of the pathogen by traditional means to approval of a vaccine based on a double-blind placebo-controlled trial.[129]
Initial vaccine sales were very slow: US Veteran's Administration expected to give out 90,000 doses to its employees, but only 30,000 doses were taken up by health care workers, who are a main risk group for occupational exposure (needlesticks, etc.).[130] The vaccine was produced from serum of homosexual chronic HBV carriers, and Dr. John Finkbeiner in January 1983 warned it "might be contaminated with a pathogen responsible for the acquired immune deficiency syndrome (AIDS) epidemic."[131] Others believed the vaccine could contribute to AIDS. In 1986, research began on a second generation of vaccines that do not use human serum, and the first vaccine was discontinued in 1990.[123]
Society and culture
World Hepatitis Day, observed 28 July, aims to raise global awareness of hepatitis B and hepatitis C and encourage prevention, diagnosis, and treatment. It has been led by the World Hepatitis Alliance since 2007 and in May 2010, it received global endorsement from the World Health Organization.[132]
See also
References
- "Hepatitis B Fact sheet N°204". who.int. July 2014. Archived from the original on 9 November 2014. Retrieved 4 November 2014.
- Chang MH (June 2007). "Hepatitis B virus infection". Semin Fetal Neonatal Med. 12 (3): 160–167. doi:10.1016/j.siny.2007.01.013. PMID 17336170.
- GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- "Hepatitis B FAQs for the Public – Transmission". U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 11 December 2011. Retrieved 29 November 2011.
- GBD 2017 Disease and Injury Incidence and Prevalence, Collaborators. (10 November 2018). "Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet. 392 (10159): 1789–1858. doi:10.1016/S0140-6736(18)32279-7. PMC 6227754. PMID 30496104.
- GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- Logan CM, Rice MK (1987). Logan's Medical and Scientific Abbreviations. J. B. Lippincott and Company. pp. 232. ISBN 0-397-54589-4.
- "Hepatitis MedlinePlus". U.S. National Library of Medicine. Retrieved 19 June 2020.
- Rubin R, Strayer DS (2008). Rubin's Pathology : clinicopathologic foundations of medicine (5th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 638. ISBN 9780781795166.
- Thomas HC (2013). Viral Hepatitis (4th ed.). Hoboken: Wiley. p. 83. ISBN 9781118637302.
- Global hepatitis report 2017 (PDF). WHO. 2017. ISBN 978-92-4-156545-5.
- Pungpapong S, Kim WR, Poterucha JJ (2007). "Natural History of Hepatitis B Virus Infection: an Update for Clinicians". Mayo Clinic Proceedings. 82 (8): 967–975. doi:10.4065/82.8.967. PMID 17673066.
- Williams R (2006). "Global challenges in liver disease". Hepatology. 44 (3): 521–526. doi:10.1002/hep.21347. PMID 16941687. S2CID 23924901.
- GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- Barker LF, Shulman NR, Murray R, Hirschman RJ, Ratner F, Diefenbach WC, Geller HM (1996). "Transmission of serum hepatitis. 1970". Journal of the American Medical Association. 276 (10): 841–844. doi:10.1001/jama.276.10.841. PMID 8769597.
- Terrault N, Roche B, Samuel D (July 2005). "Management of the hepatitis B virus in the liver transplantation setting: a European and an American perspective". Liver Transplant. 11 (7): 716–32. doi:10.1002/lt.20492. PMID 15973718. S2CID 19746065.
- El-Serag HB, Rudolph KL (June 2007). "Hepatocellular carcinoma: epidemiology and molecular carcinogenesis". Gastroenterology. 132 (7): 2557–76. doi:10.1053/j.gastro.2007.04.061. PMID 17570226.
- El-Serag HB (22 September 2011). "Hepatocellular carcinoma". New England Journal of Medicine. 365 (12): 1118–27. doi:10.1056/NEJMra1001683. PMID 21992124.
- Gan SI, Devlin SM, Scott-Douglas NW, Burak KW (October 2005). "Lamivudine for the treatment of membranous glomerulopathy secondary to chronic hepatitis B infection". Canadian Journal of Gastroenterology. 19 (10): 625–9. doi:10.1155/2005/378587. PMID 16247526.
- Dienstag JL (February 1981). "Hepatitis B as an immune complex disease". Seminars in Liver Disease. 1 (1): 45–57. doi:10.1055/s-2008-1063929. PMID 6126007.
- Trepo C, Guillevin L (May 2001). "Polyarteritis nodosa and extrahepatic manifestations of HBV infection: the case against autoimmune intervention in pathogenesis". Journal of Autoimmunity. 16 (3): 269–74. doi:10.1006/jaut.2000.0502. PMID 11334492.
- Alpert E, Isselbacher KJ, Schur PH (July 1971). "The pathogenesis of arthritis associated with viral hepatitis. Complement-component studies". The New England Journal of Medicine. 285 (4): 185–9. doi:10.1056/NEJM197107222850401. PMID 4996611.
- Liang TJ (May 2009). "Hepatitis B: the virus and disease". Hepatology. 49 (5 Suppl): S13–21. doi:10.1002/hep.22881. PMC 2809016. PMID 19399811.
- Gocke DJ, Hsu K, Morgan C, Bombardieri S, Lockshin M, Christian CL (December 1970). "Association between polyarteritis and Australia antigen". Lancet. 2 (7684): 1149–53. doi:10.1016/S0140-6736(70)90339-9. PMID 4098431.
- Lai KN, Li PK, Lui SF, Au TC, Tam JS, Tong KL, Lai FM (May 1991). "Membranous nephropathy related to hepatitis B virus in adults". The New England Journal of Medicine. 324 (21): 1457–63. doi:10.1056/NEJM199105233242103. PMID 2023605.
- Takekoshi Y, Tanaka M, Shida N, Satake Y, Saheki Y, Matsumoto S (November 1978). "Strong association between membranous nephropathy and hepatitis-B surface antigenaemia in Japanese children". Lancet. 2 (8099): 1065–8. doi:10.1016/S0140-6736(78)91801-9. PMID 82085. S2CID 28633855.
- "Hepatitis B FAQs for the Public". Centers for Disease Control and Prevention. Archived from the original on 20 August 2015. Retrieved 24 August 2015.
- Fairley CK, Read TR (February 2012). "Vaccination against sexually transmitted infections". Current Opinion in Infectious Diseases. 25 (1): 66–72. doi:10.1097/QCO.0b013e32834e9aeb. PMID 22143117. S2CID 13524636.
- Buddeberg F, Schimmer BB, Spahn DR (September 2008). "Transfusion-transmissible infections and transfusion-related immunomodulation" (PDF). Best Practice & Research. Clinical Anaesthesiology. 22 (3): 503–17. doi:10.1016/j.bpa.2008.05.003. PMID 18831300.
- Hughes RA (March 2000). "Drug injectors and the cleaning of needles and syringes". European Addiction Research. 6 (1): 20–30. doi:10.1159/000019005. PMID 10729739. S2CID 45638523.
- "Hepatitis B – the facts: IDEAS –Victorian Government Health Information, Australia". State of Victoria. 28 July 2009. Archived from the original on 18 September 2011. Retrieved 19 September 2009.
- Shapiro CN (May 1993). "Epidemiology of hepatitis B". Pediatr. Infect. Dis. J. 12 (5): 433–437. doi:10.1097/00006454-199305000-00036. PMID 8392167.
- Shi Z, Yang Y, Wang H, Ma L, Schreiber A, Li X, Sun W, Zhao X, Yang X, Zhang L, Lu W, Teng J, An Y (2011). "Breastfeeding of Newborns by Mothers Carrying Hepatitis B Virus: A Meta-analysis and Systematic Review". Archives of Pediatrics and Adolescent Medicine. 165 (9): 837–846. doi:10.1001/archpediatrics.2011.72. PMID 21536948.
- WHO | Hepatitis B Archived 9 November 2014 at the Wayback Machine
- Zuckerman AJ (1996). "Hepatitis Viruses". In Baron S, et al. (eds.). Baron's Medical Microbiology (4th ed.). University of Texas Medical Branch. ISBN 978-0-9631172-1-2. Archived from the original on 14 July 2009.
- Locarnini S (2004). "Molecular Virology of Hepatitis B Virus". Seminars in Liver Disease. 24: 3–10. CiteSeerX 10.1.1.618.7033. doi:10.1055/s-2004-828672. PMID 15192795.
- Harrison T (2009). Desk Encyclopedia of General Virology. Boston: Academic Press. p. 455. ISBN 978-0-12-375146-1.
- Howard CR (1986). "The Biology of Hepadnaviruses". Journal of General Virology. 67 (7): 1215–1235. doi:10.1099/0022-1317-67-7-1215. PMID 3014045.
- Kay A, Zoulim F (2007). "Hepatitis B virus genetic variability and evolution" (PDF). Virus Research. 127 (2): 164–176. doi:10.1016/j.virusres.2007.02.021. PMID 17383765.
- Buti M, Rodriguez-Frias F, Jardi R, Esteban R (December 2005). "Hepatitis B virus genome variability and disease progression: the impact of pre-core mutants and HBV genotypes". Journal of Clinical Virology. 34 Suppl 1: S79–82. doi:10.1016/s1386-6532(05)80015-0. PMID 16461229.
- Glebe D, Urban S (January 2007). "Viral and cellular determinants involved in hepadnaviral entry". World Journal of Gastroenterology. 13 (1): 22–38. doi:10.3748/wjg.v13.i1.22. PMC 4065874. PMID 17206752.
- Beck J, Nassal M (January 2007). "Hepatitis B virus replication". World Journal of Gastroenterology. 13 (1): 48–64. doi:10.3748/wjg.v13.i1.48. PMC 4065876. PMID 17206754.
- Watashi K, Wakita T (August 2015). "Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism". Cold Spring Harbor Perspectives in Medicine. 5 (8): a021378. doi:10.1101/cshperspect.a021378. PMC 4526719. PMID 26238794.
- Carter J (2013). Virology : principles and applications. Saunders, Venetia. Hoboken, N.J.: Wiley. ISBN 978-1-118-62979-6. OCLC 865013042.
- Li W, Miao X, Qi Z, Zeng W, Liang J, Liang Z (2010). "Hepatitis B virus X protein upregulates HSP90alpha expression via activation of c-Myc in human hepatocarcinoma cell line, HepG2". Virol. J. 7: 45. doi:10.1186/1743-422X-7-45. PMC 2841080. PMID 20170530.
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W (2012). "Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus". eLife. 1: e00049. doi:10.7554/eLife.00049. PMC 3485615. PMID 23150796.
- Bruss V (January 2007). "Hepatitis B virus morphogenesis". World J. Gastroenterol. 13 (1): 65–73. doi:10.3748/wjg.v13.i1.65. PMC 4065877. PMID 17206755.
- Kramvis A, Kew M, François G (March 2005). "Hepatitis B virus genotypes". Vaccine. 23 (19): 2409–23. doi:10.1016/j.vaccine.2004.10.045. PMID 15752827.
- Magnius LO, Norder H (1995). "Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene". Intervirology. 38 (1–2): 24–34. doi:10.1159/000150411. PMID 8666521.
- Araujo, NM (December 2015). "Hepatitis B virus intergenotypic recombinants worldwide: An overview". Infection, Genetics and Evolution. 36: 500–10. doi:10.1016/j.meegid.2015.08.024. PMID 26299884.
- Mohsen RT, Al-azzawi RH, Ad'hiah AH (2019). "Hepatitis B virus genotypes among chronic hepatitis B patients from Baghdad, Iraq and their impact on liver function". Gene Reports. 17: 100548. doi:10.1016/j.genrep.2019.100548.
- Norder H, Couroucé AM, Magnius LO (1994). "Complete genomes, phylogenic relatedness and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes". Virology. 198 (2): 489–503. doi:10.1006/viro.1994.1060. PMID 8291231.
- Shibayama T, Masuda G, Ajisawa A, Hiruma K, Tsuda F, Nishizawa T, Takahashi M, Okamoto H (May 2005). "Characterization of seven genotypes (A to E, G and H) of hepatitis B virus recovered from Japanese patients infected with human immunodeficiency virus type 1". Journal of Medical Virology. 76 (1): 24–32. doi:10.1002/jmv.20319. PMID 15779062. S2CID 25288772.
- Schaefer S (January 2007). "Hepatitis B virus taxonomy and hepatitis B virus genotypes". World Journal of Gastroenterology. 13 (1): 14–21. doi:10.3748/wjg.v13.i1.14. PMC 4065870. PMID 17206751.
- Tong S, Li J, Wands JR (1999). "Carboxypeptidase D is an avian hepatitis B virus receptor". Journal of Virology. 73 (10): 8696–8702. doi:10.1128/JVI.73.10.8696-8702.1999. PMC 112890. PMID 10482623.
- Glebe D, Urban S (January 2007). "Viral and cellular determinants involved in hepadnaviral entry". World J. Gastroenterol. 13 (1): 22–38. doi:10.3748/wjg.v13.i1.22. PMC 4065874. PMID 17206752.
- Coffin CS, Mulrooney-Cousins PM, van Marle G, Roberts JP, Michalak TI, Terrault NA (April 2011). "Hepatitis B virus (HBV) quasispecies in hepatic and extrahepatic viral reservoirs in liver transplant recipients on prophylactic therapy". Liver Transpl. 17 (8): 955–62. doi:10.1002/lt.22312. PMID 21462295. S2CID 206211853.
- Iannacone M, Sitia G, Ruggeri ZM, Guidotti LG (2007). "HBV pathogenesis in animal models: Recent advances on the role of platelets". Journal of Hepatology. 46 (4): 719–726. doi:10.1016/j.jhep.2007.01.007. PMC 1892635. PMID 17316876.
- Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG (November 2005). "Platelets mediate cytotoxic T lymphocyte-induced liver damage". Nat. Med. 11 (11): 1167–9. doi:10.1038/nm1317. PMC 2908083. PMID 16258538.
- Bonino F, Chiaberge E, Maran E, Piantino P (1987). "Serological markers of HBV infectivity". Ann. Ist. Super. Sanità. 24 (2): 217–23. PMID 3331068.
- Karayiannis P, Thomas HC (2009). Mahy BW, van Regenmortel MH (eds.). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. p. 110. ISBN 978-0-12-375147-8.
- Liaw YF, Brunetto MR, Hadziyannis S (2010). "The natural history of chronic HBV infection and geographical differences". Antiviral Therapy. 15: 25–33. doi:10.3851/IMP1621. PMID 21041901.
- Lok AS, McMahon BJ (February 2007). "Chronic hepatitis B". Hepatology. 45 (2): 507–39. doi:10.1002/hep.21513. hdl:2027.42/55941. PMID 17256718. S2CID 8713169.
- Chu CM, Liaw YF (November 2007). "Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B". Gastroenterology. 133 (5): 1458–65. doi:10.1053/j.gastro.2007.08.039. PMID 17935720.
- Zoulim F (November 2006). "New nucleic acid diagnostic tests in viral hepatitis". Semin. Liver Dis. 26 (4): 309–317. doi:10.1055/s-2006-951602. PMID 17051445.
- Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, et al. (December 2013). "CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management". MMWR. Recommendations and Reports. 62 (RR-10): 1–19. PMID 24352112. Archived from the original on 19 June 2017.
- COMMITTEE ON INFECTIOUS DISEASES; COMMITTEE ON FETUS AND NEWBORN (September 2017). "Elimination of Perinatal Hepatitis B: Providing the First Vaccine Dose Within 24 Hours of Birth". Pediatrics. 140 (3): e20171870. doi:10.1542/peds.2017-1870. PMID 28847980.
- Chan SL, Wong VW, Qin S, Chan HL (January 2016). "Infection and Cancer: The Case of Hepatitis B". Journal of Clinical Oncology. 34 (1): 83–90. doi:10.1200/JCO.2015.61.5724. PMID 26578611.
- Lee C, Gong Y, Brok J, Boxall EH, Gluud C (April 2006). "Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers". The Cochrane Database of Systematic Reviews (2): CD004790. doi:10.1002/14651858.CD004790.pub2. PMID 16625613.
- Wong F, Pai R, Van Schalkwyk J, Yoshida EM (2014). "Hepatitis B in pregnancy: a concise review of neonatal vertical transmission and antiviral prophylaxis". Annals of Hepatology. 13 (2): 187–95. doi:10.1016/S1665-2681(19)30881-6. PMID 24552860.
- Hyun MH, Lee YS, Kim JH, Je JH, Yoo YJ, Yeon JE, Byun KS (June 2017). "Systematic review with meta-analysis: the efficacy and safety of tenofovir to prevent mother-to-child transmission of hepatitis B virus". Alimentary Pharmacology & Therapeutics. 45 (12): 1493–1505. doi:10.1111/apt.14068. PMID 28436552. S2CID 23620357.
- Eke AC, Eleje GU, Eke UA, Xia Y, Liu J (February 2017). "Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus". The Cochrane Database of Systematic Reviews. 2: CD008545. doi:10.1002/14651858.CD008545.pub2. PMC 6464495. PMID 28188612.
- Sangkomkamhang US, Lumbiganon P, Laopaiboon M (November 2014). "Hepatitis B vaccination during pregnancy for preventing infant infection". The Cochrane Database of Systematic Reviews (11): CD007879. doi:10.1002/14651858.CD007879.pub3. PMC 7185858. PMID 25385500.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP (2006). "Hepatitis B virus infection: epidemiology and vaccination". Epidemiologic Reviews. 28: 112–25. doi:10.1093/epirev/mxj009. PMID 16754644.
- Chen W, Gluud C (October 2005). "Vaccines for preventing hepatitis B in health-care workers". The Cochrane Database of Systematic Reviews (4): CD000100. doi:10.1002/14651858.CD000100.pub3. PMID 16235273.
- Schroth RJ, Hitchon CA, Uhanova J, Noreddin A, Taback SP, Moffatt ME, Zacharias JM (19 July 2004). "Hepatitis B vaccination for patients with chronic renal failure". The Cochrane Database of Systematic Reviews (3): CD003775. doi:10.1002/14651858.CD003775.pub2. PMID 15266500.
- "Men Who Have Sex with Men | Populations and Settings | Division of Viral Hepatitis | CDC". www.cdc.gov. 31 May 2015. Retrieved 13 December 2017.
- Lutgens SP, Nelissen EC, van Loo IH, Koek GH, Derhaag JG, Dunselman GA (22 July 2009). "To do or not to do: IVF and ICSI in chronic hepatitis B virus carriers". Human Reproduction. 24 (11): 2676–8. doi:10.1093/humrep/dep258. PMID 19625309.
- LeFevre ML (July 2014). "Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement". Annals of Internal Medicine. 161 (1): 58–66. doi:10.7326/M14-1018. PMID 24863637.
- Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. (July 2019). "Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force Reaffirmation Recommendation Statement". JAMA. 322 (4): 349–354. doi:10.1001/jama.2019.9365. PMID 31334800.
- Hollinger FB, Lau DT (December 2006). "Hepatitis B: the pathway to recovery through treatment". Gastroenterology Clinics of North America. 35 (4): 895–931. doi:10.1016/j.gtc.2006.10.002. PMID 17129820.(registration required)
- HBV FAQs for Health Professionals | Division of Viral Hepatitis | CDC Archived 20 August 2017 at the Wayback Machine
- Lai CL, Yuen MF (July 2007). "The natural history and treatment of chronic hepatitis B: a critical evaluation of standard treatment criteria and end points". Annals of Internal Medicine. 147 (1): 58–61. doi:10.7326/0003-4819-147-1-200707030-00010. PMID 17606962. S2CID 40746103.
- Alberti A, Caporaso N (January 2011). "HBV therapy: guidelines and open issues". Digestive and Liver Disease. 43 Suppl 1 (Suppl 1): S57-63. doi:10.1016/S1590-8658(10)60693-7. PMID 21195373.
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH (January 2016). "AASLD guidelines for treatment of chronic hepatitis B". Hepatology. 63 (1): 261–83. doi:10.1002/hep.28156. PMC 5987259. PMID 26566064.
- GUIDELINES FOR THE PREVENTION, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS B INFECTION (PDF). World Health Organization. March 2015. ISBN 978924154905-9. Archived (PDF) from the original on 19 March 2015.
- Dienstag JL (2008). "Hepatitis B Virus Infection". New England Journal of Medicine. 359 (14): 1486–1500. doi:10.1056/NEJMra0801644. PMID 18832247.
- Pramoolsinsup C (February 2002). "Management of viral hepatitis B". Journal of Gastroenterology and Hepatology. 17 (Suppl): S125–45. doi:10.1046/j.1440-1746.17.s1.3.x. PMID 12000599. S2CID 26270129.(subscription required)
- Cao GW (December 2009). "Clinical relevance and public health significance of hepatitis B virus genomic variations". World Journal of Gastroenterology. 15 (46): 5761–9. doi:10.3748/wjg.15.5761. PMC 2791267. PMID 19998495. Archived from the original on 29 June 2011.
- Bell SJ, Nguyen T (2009). "The management of hepatitis B". Aust Prescr. 32 (4): 99–104. doi:10.18773/austprescr.2009.048.
- Kerkar N (2005). "Hepatitis B in children: complexities in management". Pediatric Transplantation. 9 (5): 685–691. doi:10.1111/j.1399-3046.2005.00393.x. PMID 16176431. S2CID 6437448.
- Taylor JM (2006). "Hepatitis delta virus". Virology. 344 (1): 71–76. doi:10.1016/j.virol.2005.09.033. PMID 16364738.
- Oliveri F, Brunetto MR, Actis GC, Bonino F (November 1991). "Pathobiology of chronic hepatitis virus infection and hepatocellular carcinoma (HCC)". Ital J Gastroenterol. 23 (8): 498–502. PMID 1661197.
- Peters MG (January 2019). "Hepatitis B Virus Infection: What Is Current and New". Topics in Antiviral Medicine. 26 (4): 112–116. PMC 6372357. PMID 30641484.
- Vierling JM (November 2007). "The immunology of hepatitis B". Clin Liver Dis. 11 (4): 727–759, vii–759. doi:10.1016/j.cld.2007.08.001. PMID 17981227.
- Villa E, Fattovich G, Mauro A, Pasino M (January 2011). "Natural history of chronic HBV infection: special emphasis on the prognostic implications of the inactive carrier state versus chronic hepatitis". Digestive and Liver Disease. 43 (Suppl 1): S8–14. doi:10.1016/S1590-8658(10)60686-X. PMID 21195374.
- Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-Kaspa R (February 2008). "Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis". J. Viral Hepat. 15 (2): 89–102. doi:10.1111/j.1365-2893.2007.00902.x. PMID 18184191. S2CID 37659362.
- Roche B, Samuel D (January 2011). "The difficulties of managing severe hepatitis B virus reactivation". Liver International. 31 (Suppl 1): 104–10. doi:10.1111/j.1478-3231.2010.02396.x. PMID 21205146. S2CID 19400774.
- Mastroianni CM, Lichtner M, Citton R, Del Borgo C, Rago A, Martini H, Cimino G, Vullo V (September 2011). "Current trends in management of hepatitis B virus reactivation in the biologic therapy era". World Journal of Gastroenterology. 17 (34): 3881–7. doi:10.3748/wjg.v17.i34.3881. PMC 3198017. PMID 22025876.
- Bonacini, Maurizio, MD. "Hepatitis B Reactivation". University of Southern California Department of Surgery. Archived from the original on 27 November 2008. Retrieved 24 January 2009.
- "Hepatitis B incidence rate". Our World in Data. Retrieved 5 March 2020.
- "Hepatitis B". www.who.int. Retrieved 20 April 2020.
- Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV (November–December 2004). "Global epidemiology of hepatitis B virus". Journal of Clinical Gastroenterology. 38 (10 Suppl 3): S158–68. doi:10.1097/00004836-200411003-00008. PMID 15602165. S2CID 39206739.
- Redd JT, Baumbach J, Kohn W, Nainan O, Khristova M, Williams I (May 2007). "Patient-to-patient transmission of hepatitis B virus associated with oral surgery". J. Infect. Dis. 195 (9): 1311–4. doi:10.1086/513435. PMID 17397000.
- Alter MJ (2003). "Epidemiology and prevention of hepatitis B". Seminars in Liver Disease. 23 (1): 39–46. doi:10.1055/s-2003-37583. PMID 12616449.
- Komas NP, Vickos U, Hübschen JM, Béré A, Manirakiza A, Muller CP, Le Faou A (1 January 2013). "Cross-sectional study of hepatitis B virus infection in rural communities, Central African Republic". BMC Infectious Diseases. 13: 286. doi:10.1186/1471-2334-13-286. PMC 3694350. PMID 23800310.
- "Healthcare stumbling in RI's Hepatitis fight". The Jakarta Post. 13 January 2011. Archived from the original on 4 March 2016.
- Mühlemann B, Jones TC, Damgaard PB, Allentoft ME, Shevnina I, Logvin A, et al. (May 2018). "Ancient hepatitis B viruses from the Bronze Age to the Medieval period". Nature. 557 (7705): 418–423. Bibcode:2018Natur.557..418M. doi:10.1038/s41586-018-0097-z. PMID 29743673. S2CID 13684815.
- Ben Guarino (9 May 2018). "New strains of hepatitis B virus discovered in ancient human remains". The Washington Post. Retrieved 9 January 2018.
- Patterson Ross Z, Klunk J, Fornaciari G, Giuffra V, Duchêne S, Duggan AT, et al. (January 2018). "The paradox of HBV evolution as revealed from a 16th century mummy". PLOS Pathogens. 14 (1): e1006750. doi:10.1371/journal.ppat.1006750. PMC 5754119. PMID 29300782.
- Davis, Jane (2013). "Molecular Epidemiology of Hepatitis B in the Indigenous People of Northern Australia". Gastroenterology and Hepatology. 2013 July (7): 1234–41. doi:10.1111/jgh.12177. PMID 23432545. S2CID 5208526.
- Gerlich, Wolfram (2013). "Medical Virology of Hepatitis B: how it began and where we are now". Virology Journal. 2013, 10: 239. doi:10.1186/1743-422X-10-239. PMC 3729363. PMID 23870415.
- Paraskevis, Dimitrios (2013). "Dating the Origin and Dispersal of Hepatitis B Virus Infection in Humans and Primates". Hepatology. 2013 (3): 908–16. doi:10.1002/hep.26079. PMID 22987324. S2CID 25933906.
- Lurman A (1885). "Eine icterus epidemic". Berl Klin Woschenschr (in German). 22: 20–3.
- "World War II Hepatitis Outbreak Was Biggest in History". Associated Press. Boston. 16 April 1987. Retrieved 8 November 2020.
- Jacobsen, Annie (2014). Operation Paperclip: The Secret Intelligence Program that Brought Nazi Scientists to America. p. 6. ISBN 978-0-316-22104-7.
- "Report: Yale professor intentionally gave patients hepatitis in 1940s study". CT Post. 1 March 2011. Retrieved 8 November 2020.
- Jakubik A, Ryn Z (1973). "Pseudomedical experiments in Nazi concentration camps". Przegl Lek. 30 (1): 64–72. PMID 4571138. Retrieved 8 November 2020.
- "Nazi Medical Experiments". Holocaust Encyclopedia. United States Holocaust Memorial Museum. Retrieved 8 November 2020.
- Alter HJ, Blumberg BS (March 1966). "Further studies on a "new" human isoprecipitin system (Australia antigen)". Blood. 27 (3): 297–309. doi:10.1182/blood.V27.3.297.297. PMID 5930797.
- MacCallum FO (1947). "Homologous serum hepatitis". Lancet. 2 (6480): 691–692. doi:10.1016/S0140-6736(47)90722-8.
- Dane DS, Cameron CH, Briggs M (April 1970). "Virus-like particles in serum of patients with Australia-antigen-associated hepatitis". Lancet. 1 (7649): 695–8. doi:10.1016/S0140-6736(70)90926-8. PMID 4190997.
- "Hepatitis B Vaccine History". Hepatitis B Foundation. Retrieved 8 November 2020.
- Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P (October 1979). "Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli". Nature. 281 (5733): 646–50. Bibcode:1979Natur.281..646G. doi:10.1038/281646a0. PMID 399327.
- Dietzman D, Harnisch J, Ray G, Alexander R, Holmes K (1977). "Hepatitis B surfice antigen (HBsAG) and antibody to HBsAG. Prevalence in homosexual and heterosexual men". JAMA. 238 (24): 2625–6. doi:10.1001/jama.1977.03280250051022. PMID 579199.
- Goodfield, June (1985). Quest for the Killers. Hill and Wang. pp. 51–97.
- Szmuness W, Stevens C, Harley E, et al. (1980). "Szmuness chooses healthy young homosexuals for his experiment". The New England Journal of Medicine. 303 (15): 833–41. doi:10.1056/NEJM198010093031501. PMID 6997738.
- Francis, Don (1982). "The prevention of hepatitis B with vaccine. Report of the Centers for Disease Control multi-center efficacy trial among homosexual men". Annals of Internal Medicine. 97 (3): 362–366. doi:10.7326/0003-4819-97-3-362. PMID 6810736.
- Goodfield, June. Quest for the Killers. p. 94.
- Rappoport, John (2004). AIDS, Inc. Namaste Publishing. p. 328. ISBN 0-9546590-1-5.
- Martin, Noreen (2007). Surviving AIDS and Cancer: A Guide to Staying Healthy. iUniverse. p. 28. ISBN 9780595431526. Retrieved 11 November 2020.
- "Viral hepatitis" (PDF). World Health Organization. Archived (PDF) from the original on 11 August 2011.
External links
| Classification | |
|---|---|
| External resources |
| Wikimedia Commons has media related to Hepatitis B. |
- GUIDELINES FOR THE PREVENTION, CARE AND TREATMENT OF PERSONS WITH CHRONIC HEPATITIS B INFECTION (PDF). World Health Organization. March 2015. ISBN 978924154905-9.
- "Hepatitis B virus". NCBI Taxonomy Browser. 10407.