Ankylosing spondylitis
Ankylosing spondylitis (AS) is a type of arthritis in which there is a long-term inflammation of the joints of the spine.[2] Typically the joints where the spine joins the pelvis are also affected.[2] Occasionally other joints such as the shoulders or hips are involved.[2] Eye and bowel problems may also occur.[2] Back pain is a characteristic symptom of AS, and it often comes and goes.[2] Stiffness of the affected joints generally worsens over time.[2][4]
| Ankylosing spondylitis | |
|---|---|
| Other names | Bekhterev's disease, Bechterew's disease, morbus Bechterew, Bekhterev–Strümpell–Marie disease, Marie's disease, Marie–Strümpell arthritis, Pierre–Marie's disease[1] |
_anagoria.JPG.webp) | |
| A 6th-century skeleton showing fused vertebrae, a sign of severe ankylosing spondylitis | |
| Specialty | Rheumatology |
| Symptoms | Back pain, joint stiffness[2] |
| Usual onset | Young adulthood[2] |
| Duration | Long term[2] |
| Causes | Unknown[2] |
| Diagnostic method | Symptom based, medical imaging, blood tests[2] |
| Treatment | Medication, exercise, physical therapy |
| Medication | NSAIDs, steroids, DMARDs,[2] TNF Inhibitor |
| Frequency | 0.1 to 0.8%[3] |
Although the cause of ankylosing spondylitis is unknown, it is believed to involve a combination of genetic and environmental factors.[2] More than 85% of those affected in the UK have a specific human leukocyte antigen known as the HLA-B27 antigen.[5] The underlying mechanism is believed to be autoimmune or autoinflammatory.[6] Diagnosis is typically based on the symptoms with support from medical imaging and blood tests.[2] AS is a type of seronegative spondyloarthropathy, meaning that tests show no presence of rheumatoid factor (RF) antibodies.[2] It is also within a broader category known as axial spondyloarthritis.[7]
There is no cure for ankylosing spondylitis.[2] Treatments may improve symptoms and prevent worsening.[2] This may include medication, exercise, physical therapy, surgery in rare cases.[2] Medications used include NSAIDs, steroids, DMARDs such as sulfasalazine, and biologic agents such as TNF inhibitors.[2]
Between 0.1% and 0.8% of people are affected.[3] Onset is typically in young adults.[2] Males and females are equally affected. It used to be thought that three times as many men as women had the disease. This was based on a diagnosis of the disease using x-ray. Men are more likely than women to experience changes to the bones and fusion, and thus they were being picked up using x-ray. Over time MRI’s were developed which could identify inflammation. Women are more likely than men to experience inflammation rather than fusion.[8] The condition was first fully described in the late 1600s by Bernard Connor, but skeletons with ankylosing spondylitis are found in Egyptian mummies.[9] The word is from Greek ankylos meaning crooked, curved or rounded, spondylos meaning vertebra, and -itis meaning inflammation.[2]
Signs and symptoms

The signs and symptoms of ankylosing spondylitis often appear gradually, with peak onset being between 20 and 30 years of age.[10] Initial symptoms are usually a chronic dull pain in the lower back or gluteal region combined with stiffness of the lower back.[11] Individuals often experience pain and stiffness that awakens them in the early morning hours.[10]
As the disease progresses, loss of spinal mobility and chest expansion, with a limitation of anterior flexion, lateral flexion, and extension of the lumbar spine, are seen. Systemic features are common, with weight loss, fever, or fatigue often present.[10] Pain is often severe at rest but may improve with physical activity, but inflammation and pain to varying degrees may recur regardless of rest and movement.
AS can occur in any part of the spine or the entire spine, often with pain referred to one or the other buttock or the back of the thigh from the sacroiliac joint. Arthritis in the hips and shoulders may also occur. When the condition presents before the age of 18, it is more likely to cause pain and swelling of large lower limb joints, such as the knees.[12] In prepubescent cases, pain and swelling may also manifest in the ankles and feet where heel pain and enthesopathy commonly develop.[12] Less commonly ectasia of the sacral nerve root sheaths may occur.[13]
About 30% of people with AS will also experience anterior uveitis, causing eye pain, redness, and blurred vision. This is thought to be due to the association that both AS and uveitis have with the inheritance of the HLA-B27 antigen.[14] Cardiovascular involvement may include inflammation of the aorta, aortic valve insufficiency or disturbances of the heart's electrical conduction system. Lung involvement is characterized by progressive fibrosis of the upper portion of the lung.[15]
Pathophysiology

Ankylosing spondylitis (AS) is a systemic rheumatic disease, meaning it affects the entire body. 1–2% of individuals with the HLA-B27 genotype develop the disease.[16] Approximately 85% of people with AS express the HLA-B27 genotype, meaning there is a strong genetic association. Tumor necrosis factor-alpha (TNF α) and IL-1 are also implicated in ankylosing spondylitis. Autoantibodies specific for AS have not been identified. Anti-neutrophil cytoplasmic antibodies (ANCAs) are associated with AS, but do not correlate with disease severity.[17]
Single nucleotide polymorphism (SNP) A/G variant rs10440635[18] close to the PTGER4 gene on human chromosome 5 has been associated with an increased number of cases of ankylosing spondylitis in a population recruited from the United Kingdom, Australia, and Canada. The PTGER4 gene codes for the prostaglandin EP4 receptor (EP4), one of four receptors for prostaglandin E2. Activation of EP4 promotes bone remodeling and deposition (see EP4, bone) and EP4 is highly expressed at vertebral column sites involved in ankylosing spondylitis. These findings suggest that excessive EP4 activation contributes to pathological bone remodeling and deposition in ankylosing spondylitis and that the A/G variant rs10440635a of PTGER4 predisposes to this disease, possibly by influencing EP4's production or expression pattern.[19][20]
The association of AS with HLA-B27 suggests the condition involves CD8 T cells, which interact with HLA-B.[21] This interaction is not proven to involve a self-antigen, and at least in the related reactive arthritis, which follows infections, the antigens involved are likely to be derived from intracellular microorganisms.[22] There is, however, a possibility that CD4+ T lymphocytes are involved in an aberrant way, since HLA-B27 appears to have a number of unusual properties, including possibly an ability to interact with T cell receptors in association with CD4 (usually CD8+ cytotoxic T cell with HLAB antigen as it is a MHC class 1 antigen).
"Bamboo spine" develops when the outer fibers of the fibrous ring (anulus fibrosus disci intervertebralis) of the intervertebral discs ossify, which results in the formation of marginal syndesmophytes between adjoining vertebrae.
Diagnosis

Ankylosing spondylitis is a member of the more broadly defined disease axial spondyloarthritis.[23] Axial spondyloarthritis can be divided into (1) radiographic axial spondyloarthritis (which is a synonym for ankylosing spondylitis) and (2) non-radiographic axial spondyloarthritis (which include less severe forms and early stages of ankylosing spondylitis) [23]
While ankylosing spondylitis can be diagnosed through the description of radiological changes in the sacroiliac joints and spine, there are currently no direct tests (blood or imaging) to unambiguously diagnose early forms of ankylosing spondylitis (non-radiographic axial spondyloarthritis). Diagnosis of non-radiologic axial spondyloarthritis is therefore more difficult and is based on the presence of several typical disease features.[23][24]
These diagnostic criteria include:
- Inflammatory back pain:
Chronic, inflammatory back pain is defined when at least four out of five of the following parameters are present: (1) Age of onset below 40 years old, (2) insidious onset, (3) improvement with exercise, (4) no improvement with rest, and (5) pain at night (with improvement upon getting up) - Past history of inflammation in the joints, heels, or tendon-bone attachments
- Family history for axial spondyloarthritis or other associated rheumatic/autoimmune conditions
- Positive for the biomarker HLA-B27
- Good response to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs)
- Signs of elevated inflammation (C-reactive protein and erythrocyte sedimentation rate)
- Manifestation of psoriasis, inflammatory bowel disease, or inflammation of the eye (uveitis)
If these criteria still do not give a compelling diagnosis magnetic resonance imaging (MRI) may be useful.[23][24] MRI can show inflammation of the sacroiliac joint.
X-rays
The earliest changes demonstrable by plain x–ray shows erosions and sclerosis in sacroiliac joints. Progression of the erosions leads to widening of the joint space and bony sclerosis. X-ray spine can reveal squaring of vertebrae with bony spur formation called syndesmophyte. This causes the bamboo spine appearance. A drawback of X-ray diagnosis is the signs and symptoms of AS have usually been established as long as 7–10 years prior to X-ray-evident changes occurring on a plain film X-ray, which means a delay of as long as 10 years before adequate therapies can be introduced.[25]
Options for earlier diagnosis are tomography and MRI of the sacroiliac joints, but the reliability of these tests is still unclear.
 Lateral X-ray of the mid back in ankylosing spondylitis
Lateral X-ray of the mid back in ankylosing spondylitis Lateral X-ray of the neck in ankylosing spondylitisImaging
Lateral X-ray of the neck in ankylosing spondylitisImaging X-ray showing bamboo spine in a person with ankylosing spondylitis
X-ray showing bamboo spine in a person with ankylosing spondylitis CT scan showing bamboo spine in ankylosing spondylitis
CT scan showing bamboo spine in ankylosing spondylitis T1-weighted MRI with fat suppression after administration of gadolinium contrast showing sacroiliitis in a person with ankylosing spondylitis
T1-weighted MRI with fat suppression after administration of gadolinium contrast showing sacroiliitis in a person with ankylosing spondylitis
Blood parameters
During acute inflammatory periods, people with AS may show an increase in the blood concentration of CRP and an increase in the ESR, but there are many with AS whose CRP and ESR rates do not increase, so normal CRP and ESR results do not always correspond with the amount of inflammation that is actually present. In other words, some people with AS have normal levels of CRP and ESR, despite experiencing a significant amount of inflammation in their bodies.[26]
Genetic testing
Variations of the HLA-B gene increase the risk of developing ankylosing spondylitis, although it is not a diagnostic test. Those with the HLA-B27 variant are at a higher risk than the general population of developing the disorder. HLA-B27, demonstrated in a blood test, can occasionally help with diagnosis, but in itself is not diagnostic of AS in a person with back pain. Over 85% of people that have been diagnosed with AS are HLA-B27 positive, although this ratio varies from population to population (about 50% of African Americans with AS possess HLA-B27 in contrast to the figure of 80% among those with AS who are of Mediterranean descent).[27]
BASDAI
The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), developed in Bath (UK), is an index designed to detect the inflammatory burden of active disease. The BASDAI can help to establish a diagnosis of AS in the presence of other factors such as HLA-B27 positivity, persistent buttock pain which resolves with exercise, and X-ray or MRI-evident involvement of the sacroiliac joints.[28] It can be easily calculated and accurately assesses the need for additional therapy; a person with AS with a score of four out of a possible 10 points while on adequate NSAID therapy is usually considered a good candidate for biologic therapy.
The Bath Ankylosing Spondylitis Functional Index (BASFI) is a functional index which can accurately assess functional impairment due to the disease, as well as improvements following therapy.[29] The BASFI is not usually used as a diagnostic tool, but rather as a tool to establish a current baseline and subsequent response to therapy.
Children
Juvenile ankylosing spondylitis (JAS) is a rare form of the disease which differs from the more common adult form.[12] Enthesophathy and arthritis of large joints of the lower extremities is more common than the characteristic early-morning back pain seen in adult AS.[12] Ankylosing tarsitis of the ankle is a common feature, as is the more classical findings of seronegative ANA and RF as well as presence of the HLA-B27 allele.[12] Primary engagement of the appendicular joints may explain delayed diagnosis, however other common symptoms of AS such as uveitis, diarrhea, pulmonary disease and heart valve disease may lead suspicion away from other juvenile spondyloarthropathies.[12]
Schober's test
The Schober's test is a useful clinical measure of flexion of the lumbar spine performed during the physical examination.[30]
Treatment
There is no cure for AS, although treatments and medications can reduce symptoms and pain.
Medication
The major types of medications used to treat ankylosing spondylitis are pain-relievers and drugs aimed at stopping or slowing the progression of the disease. All of these have potentially serious side effects. Pain-relieving drugs come in two major classes:
- The mainstay of therapy in all seronegative spondyloarthropathies are anti-inflammatory drugs, which include NSAIDs such as ibuprofen, phenylbutazone, diclofenac, indomethacin, naproxen and COX-2 inhibitors, which reduce inflammation and pain. 2012 research showed that those with AS and elevated levels of acute phase reactants seem to benefit most from continuous treatment with NSAIDs.[31]
Medications used to treat the progression of the disease include the following:
- Disease-modifying antirheumatic drugs (DMARDs) such as sulfasalazine can be used in people with peripheral arthritis. For axial involvement, evidence does not support sulfasalazine.[32] Other DMARDS, such as methotrexate, did not have enough evidence to prove their effect. Generally, systemic corticosteroids were not used due to lack of evidence. Local injection with corticosteroid can be used for certain people with peripheral arthritis.[33][34]
- Tumor necrosis factor-alpha (TNFα) blockers (antagonists), such as the biologics etanercept, infliximab, golimumab and adalimumab, have shown good short-term effectiveness in the form of profound and sustained reduction in all clinical and laboratory measures of disease activity.[35] Trials are ongoing to determine their long-term effectiveness and safety.[36] The major drawback is the cost. An alternative may be the newer, orally-administered non-biologic apremilast, which inhibits TNF-α secretion, but a recent study did not find the drug useful for ankylosing spondylitis.[37]
- Anti-interleukin-6 inhibitors such as tocilizumab, currently approved for the treatment of rheumatoid arthritis,[38] and rituximab, a monoclonal antibody against CD20, are also undergoing trials.[39]
- Interleukin-17A inhibitor secukinumab is an option for the treatment of active ankylosing spondylitis that has responded inadequately to (TNFα) blockers.[40]
Surgery
In severe cases of AS, surgery can be an option in the form of joint replacements, particularly in the knees and hips. Surgical correction is also possible for those with severe flexion deformities (severe downward curvature) of the spine, particularly in the neck, although this procedure is considered very risky. However there has been one successful operation in China on a 46-year-old man. [41] In addition, AS can have some manifestations which make anesthesia more complex. Changes in the upper airway can lead to difficulties in intubating the airway, spinal and epidural anesthesia may be difficult owing to calcification of ligaments, and a small number of people have aortic insufficiency. The stiffness of the thoracic ribs results in ventilation being mainly diaphragm-driven, so there may also be a decrease in pulmonary function.
Physical therapy
Though physical therapy remedies have been scarcely documented, some therapeutic exercises are used to help manage lower back, neck, knee, and shoulder pain. Some therapeutic exercises include:[42][43]
- Low intensity aerobic exercise
- Transcutaneous electrical nerve stimulation (TENS)
- Thermotherapy
- Proprioceptive neuromuscular facilitation (PNF)
- Exercise programs, either at home or supervised
- Hydrotherapy
- Group exercises eg Pilates
Moderate-to-high impact exercises like jogging are generally not recommended or recommended with restrictions due to the jarring of affected vertebrae that can worsen pain and stiffness in some with AS.
Prognosis

Prognosis is related to disease severity.[10] AS can range from mild to progressively debilitating and from medically controlled to refractory. Some cases may have times of active inflammation followed by times of remission resulting in minimal disability while others never have times of remission and have acute inflammation and pain, leading to significant disability.[10] As the disease progresses, it can cause the vertebrae and the lumbosacral joint to ossify, resulting in the fusion of the spine.[44] This places the spine in a vulnerable state because it becomes one bone, which causes it to lose its range of motion as well as putting it at risk for spinal fractures. This not only limits mobility but reduces the affected person's quality of life. Complete fusion of the spine can lead to a reduced range of motion and increased pain, as well as total joint destruction which could lead to a joint replacement.[45]
Osteoporosis is common in ankylosing spondylitis, both from chronic systemic inflammation and decreased mobility resulting from AS. Over a long-term period, osteopenia or osteoporosis of the AP spine may occur, causing eventual compression fractures and a back "hump".[46] Hyperkyphosis from ankylosing spondylitis can also lead to impairment in mobility and balance, as well as impaired peripheral vision, which increases the risk of falls which can cause fracture of already-fragile vertebrae.[46] Typical signs of progressed AS are the visible formation of syndesmophytes on X-rays and abnormal bone outgrowths similar to osteophytes affecting the spine. In compression fractures of the vertebrae, paresthesia is a complication due to the inflammation of the tissue surrounding nerves.
Organs commonly affected by AS, other than the axial spine and other joints, are the heart, lungs, eyes, colon, and kidneys. Other complications are aortic regurgitation, Achilles tendinitis, AV node block, and amyloidosis.[47] Owing to lung fibrosis, chest X-rays may show apical fibrosis, while pulmonary function testing may reveal a restrictive lung defect. Very rare complications involve neurologic conditions such as the cauda equina syndrome.[47][48]
Mortality
Mortality is increased in people with AS and circulatory disease is the most frequent cause of death.[49] People with AS have an increased risk of 60% for cerebrovascular mortality, and an overall increased risk of 50% for vascular mortality.[50] About one third of those with ankylosing spondylitis have severe disease, which reduces life expectancy.[51]
As increased mortality in ankylosing spondylitis is related to disease severity, factors negatively affecting outcomes include:[49][52]
- Male sex [53]
- Plus 3 of the following in the first 2 years of disease:
- Erythrocyte sedimentation rate (ESR) >30 mm/h
- Unresponsive to NSAIDs
- Limitation of lumbar spine range of motion
- Sausage-like fingers or toes
- Oligoarthritis
- Onset <16 years old
Gait
The hunched position that often results from complete spinal fusion can have an effect on a person's gait. Increased spinal kyphosis will lead to a forward and downward shift in center of mass (COM). This shift in COM has been shown to be compensated by increased knee flexion and ankle dorsiflexion. The gait of someone with ankylosing spondylitis often has a cautious pattern because they have decreased ability to absorb shock, and they cannot see the horizon.[54]
Epidemiology
Between 0.1% and 0.8% of people are affected.[3] The disease is most common in Northern European countries, and seen least in people of Afro-Caribbean descent.[10] Although the ratio of male to female disease is reportedly 3:1,[10] many rheumatologists believe the number of women with AS is underdiagnosed, as most women tend to experience milder cases of the disease. The majority of people with AS, including 95 percent of people of European descent with the disease, express the HLA-B27 antigen[55] and high levels of immunoglobulin A (IgA) in the blood.[56]
Research
In 2007, a collaborative effort by an international team of researchers in the United Kingdom, Australia and the United States led to the discovery of two genes that also contribute to the cause of AS: ARTS-1 and IL23R. The findings were published in the November 2007 edition of Nature Genetics, a journal that emphasizes research on the genetic basis for common and complex diseases.[57] Together with HLA-B27, these two genes account for roughly 70 percent of the overall number of cases of the disease.
History
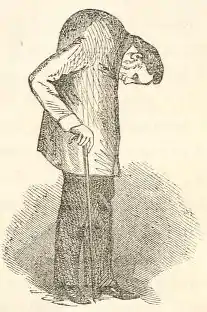
Ankylosing spondylitis has a long history, having been distinguished from rheumatoid arthritis by Galen as early as the 2nd century AD.[58] Skeletal evidence of the disease (ossification of joints and entheses primarily of the axial skeleton, known as "bamboo spine") was thought to be found in the skeletal remains of a 5000-year-old Egyptian mummy with evidence of bamboo spine.[59] However, a subsequent report found that this was not the case.[60]
The anatomist and surgeon Realdo Colombo described what could have been the disease in 1559,[61] and the first account of pathologic changes to the skeleton possibly associated with AS was published in 1691 by Bernard Connor.[62] In 1818, Benjamin Brodie became the first physician to document a person believed to have active AS who also had accompanying iritis.[63]
In 1858, David Tucker published a small booklet which clearly described the case of Leonard Trask, who suffered from severe spinal deformity subsequent to AS.[64] In 1833, Trask fell from a horse, exacerbating the condition and resulting in severe deformity. Tucker reported:
It was not until he [Trask] had exercised for some time that he could perform any labor ... [H]is neck and back have continued to curve drawing his head downward on his breast.
This account became the first documented case of AS in the United States, owing to its indisputable description of inflammatory disease characteristics of AS and the hallmark of deforming injury in AS.
In the late nineteenth century, the neurophysiologist Vladimir Bekhterev of Russia in 1893,[65] Adolph Strümpell of Germany in 1897,[66] and Pierre Marie of France in 1898[67] were the first to give adequate descriptions which permitted an accurate diagnosis of AS prior to severe spinal deformity. For this reason, AS is also known as Bekhterev disease, Bechterew's disease or Marie–Strümpell disease.
References
- Matteson EL, Woywodt A (November 2006). "Eponymophilia in rheumatology". Rheumatology. 45 (11): 1328–30. doi:10.1093/rheumatology/kel259. PMID 16920748.
- "Questions and Answers about Ankylosing Spondylitis". NIAMS. June 2016. Archived from the original on 28 September 2016. Retrieved 28 September 2016.
- Khan MA (2009). Ankylosing Spondylitis. Oxford University Press. p. 15. ISBN 9780195368079. Archived from the original on 8 September 2017.
- "Ankylosing spondylitis". GARD. 9 February 2015. Archived from the original on 2 October 2016. Retrieved 28 September 2016.
- Sheehan NJ (January 2004). "The ramifications of HLA-B27". Journal of the Royal Society of Medicine. 97 (1): 10–4. doi:10.1258/jrsm.97.1.10. PMC 1079257. PMID 14702356.
- Smith JA (January 2015). "Update on ankylosing spondylitis: current concepts in pathogenesis". Current Allergy and Asthma Reports. 15 (1): 489. doi:10.1007/s11882-014-0489-6. PMID 25447326. S2CID 24623808.
- Deodhar A, Reveille JD, van den Bosch F, Braun J, Burgos-Vargas R, Caplan L, et al. (October 2014). "The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis international Society in response to the US Food and Drug Administration's comments and concerns". Arthritis & Rheumatology. 66 (10): 2649–56. doi:10.1002/art.38776. PMID 25154344. S2CID 38228595.
- "Facts and Figures". National Axial Spondyloarthritis Society. Retrieved 27 January 2021.
- Boos N, Aebi M (2008). Spinal Disorders: Fundamentals of Diagnosis and Treatment. Springer Science & Business Media. p. 25. ISBN 9783540690917. Archived from the original on 8 September 2017.
- "Ankylosing Spondylitis -Professional reference for Doctors - Patient UK". Patient UK. Archived from the original on 7 April 2014. Retrieved 26 May 2014.
- Longo DL, Fauci AS, Harrison TR, Kasper DL, Hauser SL, Jameson JL, Loscalzo J (2012). Harrison's Principles of Internal Medicine. Vol. 1 (18th ed.). McGraw-Hill. ISBN 978-0-07-163244-7.
- Adrovic A, Barut K, Sahin S, Kasapcopur O (August 2016). "Juvenile Spondyloarthropathies". Current Rheumatology Reports. 18 (8): 55. doi:10.1007/s11926-016-0603-y. PMID 27402112. S2CID 26058238.
- Tansavatdi K (December 2020). "Dural Ectasia". Radsource.
- Cantini F, Nannini C, Cassarà E, Kaloudi O, Niccoli L (November 2015). "Uveitis in Spondyloarthritis: An Overview". The Journal of Rheumatology. Supplement. 93: 27–9. doi:10.3899/jrheum.150630. PMID 26523051. S2CID 24715271.
- Momeni M, Taylor N, Tehrani M (2011). "Cardiopulmonary manifestations of ankylosing spondylitis". International Journal of Rheumatology. 2011: 728471. doi:10.1155/2011/728471. PMC 3087354. PMID 21547038.
- "Ankylosing Spondylitis – Professional reference for Doctors – Patient UK". Patient UK. Archived from the original on 24 December 2013. Retrieved 22 December 2013.
- Rautenstrauch J, Gause A, Csernok E, Holle J, Gross WL (August 2001). "[Presentation of the Lubeck/Bad Bramstedt Competence Center]". Zeitschrift Fur Rheumatologie. 60 (4): 255–62. doi:10.1007/s003930170050. PMID 11584722.
- "Reference SNP (refSNP) Cluster Report: Rs10440635". Archived from the original on 18 February 2017. Retrieved 14 February 2017.
- Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. (July 2011). "Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility". Nature Genetics. 43 (8): 761–7. doi:10.1038/ng.873. PMC 3640413. PMID 21743469.
- Haroon N (June 2015). "Ankylosis in ankylosing spondylitis: current concepts". Clinical Rheumatology. 34 (6): 1003–7. doi:10.1007/s10067-015-2956-4. PMID 25935456. S2CID 25930196.
- Boyle LH, Goodall JC, Opat SS, Gaston JS (September 2001). "The recognition of HLA-B27 by human CD4(+) T lymphocytes". Journal of Immunology. 167 (5): 2619–24. doi:10.4049/jimmunol.167.5.2619. PMID 11509603.
- Sheehan NJ (January 2004). "The ramifications of HLA-B27". Journal of the Royal Society of Medicine. 97 (1): 10–4. doi:10.1258/jrsm.97.1.10. PMC 1079257. PMID 14702356.
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. (June 2009). "The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection". Annals of the Rheumatic Diseases. 68 (6): 777–83. doi:10.1136/ard.2009.108233. PMID 19297344.
- Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D (August 2015). "Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis". Annals of the Rheumatic Diseases. 74 (8): 1483–7. doi:10.1136/annrheumdis-2014-207151. PMID 25990288. S2CID 42585224.
- Ostergaard M, Lambert RG (August 2012). "Imaging in ankylosing spondylitis". Therapeutic Advances in Musculoskeletal Disease. 4 (4): 301–11. doi:10.1177/1759720X11436240. PMC 3403247. PMID 22859929.
- Spoorenberg A, van Tubergen A, Landewé R, Dougados M, van der Linden S, Mielants H, et al. (June 2005). "Measuring disease activity in ankylosing spondylitis: patient and physician have different perspectives". Rheumatology. 44 (6): 789–95. doi:10.1093/rheumatology/keh595. PMID 15757962.
- Maloney B (18 April 2018). "Ankylosing Spondylitis (Arthritis) / Marque Urgent Care". Marque Medical. Retrieved 25 January 2021.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (December 1994). "A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index". The Journal of Rheumatology. 21 (12): 2286–91. PMID 7699630.
- Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, Jenkinson T (December 1994). "A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index". The Journal of Rheumatology. 21 (12): 2281–5. PMID 7699629.
- Thomas E, Silman AJ, Papageorgiou AC, Macfarlane GJ, Croft PR (February 1998). "Association between measures of spinal mobility and low back pain. An analysis of new attenders in primary care". Spine. 23 (3): 343–7. doi:10.1097/00007632-199802010-00011. PMID 9507623. S2CID 41982757.
- Kroon F, Landewé R, Dougados M, van der Heijde D (October 2012). "Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis". Annals of the Rheumatic Diseases. 71 (10): 1623–9. doi:10.1136/annrheumdis-2012-201370. PMID 22532639.
- Chen J, Lin S, Liu C (November 2014). "Sulfasalazine for ankylosing spondylitis". The Cochrane Database of Systematic Reviews. 11 (11): CD004800. doi:10.1002/14651858.CD004800.pub3. PMID 25427435.
- Chen J, Veras MM, Liu C, Lin J (February 2013). "Methotrexate for ankylosing spondylitis". The Cochrane Database of Systematic Reviews. 2 (2): CD004524. doi:10.1002/14651858.CD004524.pub4. PMID 23450553.
- Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. (June 2011). "2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis". Annals of the Rheumatic Diseases. 70 (6): 896–904. doi:10.1136/ard.2011.151027. PMC 3086052. PMID 21540199.
- Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, et al. (April 2015). "TNF-alpha inhibitors for ankylosing spondylitis". The Cochrane Database of Systematic Reviews. 4 (4): CD005468. doi:10.1002/14651858.CD005468.pub2. PMID 25887212.
- Braun J, Sieper J (April 2007). "Ankylosing spondylitis". Lancet. 369 (9570): 1379–1390. doi:10.1016/S0140-6736(07)60635-7. PMID 17448825.
- Cush J. "POSTURE Study: Apremilast Fails in Ankylosing Spondylitis". Rheum Now. Archived from the original on 10 April 2017. Retrieved 10 April 2017.
- Henes JC, Horger M, Guenaydin I, Kanz L, Koetter I (December 2010). "Mixed response to tocilizumab for ankylosing spondylitis". Annals of the Rheumatic Diseases. 69 (12): 2217–8. doi:10.1136/ard.2009.126706. PMID 20525837. S2CID 31849478.
- Rodríguez-Escalera C, Fernández-Nebro A (November 2008). "The use of rituximab to treat a patient with ankylosing spondylitis and hepatitis B". Rheumatology. 47 (11): 1732–3. doi:10.1093/rheumatology/ken362. PMID 18786966.
- "Secukinumab for active Ankylosing Spondylitis". NICE. Archived from the original on 2 February 2017. Retrieved 26 January 2017.
- https://www.sznews.com/news/content/2019-12/15/content_22704729_0.htm
- "Philadelphia Panel evidence-based clinical practice guidelines on selected rehabilitation interventions: overview and methodology". Physical Therapy. 81 (10): 1629–40. October 2001. PMID 11589641.
- Dagfinrud H, Kvien TK, Hagen KB (January 2008). "Physiotherapy interventions for ankylosing spondylitis". The Cochrane Database of Systematic Reviews (1): CD002822. doi:10.1002/14651858.CD002822.pub3. PMID 18254008.
- Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A (December 2002). "Ankylosing spondylitis: an overview". Annals of the Rheumatic Diseases. 61 Suppl 3: iii8-18. doi:10.1136/ard.61.suppl_3.iii8. PMC 1766729. PMID 12381506.
- Bond D (December 2013). "Ankylosing spondylitis: diagnosis and management". Nursing Standard. 28 (16–18): 52–9, quiz 60. doi:10.7748/ns2013.12.28.16.52.e7807. PMID 24345154.
- Briot K, Roux C (2015). "Inflammation, bone loss and fracture risk in spondyloarthritis". RMD Open. 1 (1): e000052. doi:10.1136/rmdopen-2015-000052. PMC 4613172. PMID 26509065.
- Alpert JS (2006). The AHA Clinical Cardiac Consult. Lippincott Williams & Wilkins. ISBN 978-0-7817-6490-2.
- Ahn NU, Ahn UM, Nallamshetty L, Springer BD, Buchowski JM, Funches L, et al. (October 2001). "Cauda equina syndrome in ankylosing spondylitis (the CES-AS syndrome): meta-analysis of outcomes after medical and surgical treatments". Journal of Spinal Disorders. 14 (5): 427–33. doi:10.1097/00002517-200110000-00009. PMID 11586143.
- Bakland G, Gran JT, Nossent JC (November 2011). "Increased mortality in ankylosing spondylitis is related to disease activity". Annals of the Rheumatic Diseases. 70 (11): 1921–5. doi:10.1136/ard.2011.151191. PMID 21784726. S2CID 39397817.
- "Ankylosing Spondylitis Linked to Cardiovascular Mortality". Medscape. Archived from the original on 14 September 2015. Retrieved 7 October 2015.
- Braun J, Pincus T (2002). "Mortality, course of disease and prognosis of patients with ankylosing spondylitis". Clinical and Experimental Rheumatology. 20 (6 Suppl 28): S16-22. PMID 12463441. Archived from the original on 1 November 2016.
- Radford EP, Doll R, Smith PG (September 1977). "Mortality among patients with ankylosing spondylitis not given X-ray therapy". The New England Journal of Medicine. 297 (11): 572–6. doi:10.1056/NEJM197709152971103. PMID 887115.
- Lehtinen K (March 1993). "Mortality and causes of death in 398 patients admitted to hospital with ankylosing spondylitis". Annals of the Rheumatic Diseases. 52 (3): 174–6. doi:10.1136/ard.52.3.174. PMC 1005012. PMID 8484668.
- Del Din S, Carraro E, Sawacha Z, Guiotto A, Bonaldo L, Masiero S, Cobelli C (July 2011). "Impaired gait in ankylosing spondylitis". Medical & Biological Engineering & Computing. 49 (7): 801–9. doi:10.1007/s11517-010-0731-x. PMID 21229328. S2CID 17921823.
- Goldman L (2011). Goldman's Cecil Medicine (24th ed.). Philadelphia: Elsevier Saunders. p. 607. ISBN 978-1-4377-2788-3.
- Veys EM, van Leare M (November 1973). "Serum IgG, IgM, and IgA levels in ankylosing spondylitis". Annals of the Rheumatic Diseases. 32 (6): 493–6. doi:10.1136/ard.32.6.493. PMC 1006157. PMID 4202498.
- Brionez TF, Reveille JD (July 2008). "The contribution of genes outside the major histocompatibility complex to susceptibility to ankylosing spondylitis". Current Opinion in Rheumatology. 20 (4): 384–91. doi:10.1097/BOR.0b013e32830460fe. PMID 18525349. S2CID 205485848.
- Dieppe P (January 1988). "Did Galen describe rheumatoid arthritis?". Annals of the Rheumatic Diseases. 47 (1): 84–5. doi:10.1136/ard.47.1.84-b. PMC 1003452. PMID 3278697.
- Calin A (April 1985). "Ankylosing spondylitis". Clinics in Rheumatic Diseases. 11 (1): 41–60. PMID 3158467.
- Saleem SN, Hawass Z (December 2014). "Ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis in royal Egyptian mummies of 18th -20th Dynasties? CT and archaeology studies". Arthritis & Rheumatology. 66 (12): 3311–6. doi:10.1002/art.38864. PMID 25329920. S2CID 42296180.
- Benoist M (April 1995). "Pierre Marie. Pioneer investigator in ankylosing spondylitis". Spine. 20 (7): 849–52. doi:10.1097/00007632-199504000-00022. PMID 7701402.
- Blumberg BS (December 1958). "Bernard Connor's description of the pathology of ankylosing spondylitis". Arthritis and Rheumatism. 1 (6): 553–63. doi:10.1002/art.1780010609. PMID 13607268.
- Leden I (1994). "Did Bechterew describe the disease which is named after him? A question raised due to the centennial of his primary report". Scandinavian Journal of Rheumatology. 23 (1): 42–5. doi:10.3109/03009749409102134. PMID 8108667.
- "Life and sufferings of Leonard Trask" (PDF). Ankylosing Spondylitis Information Matrix. Archived (PDF) from the original on 8 July 2011.
- Bechterew W (1893). "Steifigkeit der Wirbelsaule und ihre Verkrummung als besondere Erkrankungsform". Neurol Centralbl. 12: 426–434.
- Strumpell A (1897). "Bemerkung uber die chronische ankylosirende Entzundung der Wirbelsaule und der Huftgelenke". Dtsch Z Nervenheilkd. 11 (3–4): 338–342. doi:10.1007/BF01674127. S2CID 34700673.
- Marie P (1898). "Sur la spondylose rhizomelique". Rev Med. 18: 285–315.
External links
| Classification | |
|---|---|
| External resources |
- Ankylosing spondylitis at Curlie
- Questions and Answers about Ankylosing Spondylitis - US National Institute of Arthritis and Musculoskeletal and Skin Diseases