Hospital-acquired infection
A Hospital-acquired infection also known as a nosocomial infection -from the Greek words nosos, meaning disease, and komide, care- , is an infection that is acquired in a hospital or other health care facility.[1] To emphasize both hospital and nonhospital settings, it is sometimes instead called a healthcare–associated infection.[2] Such an infection can be acquired in hospital, nursing home, rehabilitation facility, outpatient clinic, diagnostic laboratory or other clinical settings. Infection is spread to the susceptible patient in the clinical setting by various means. Health care staff also spread infection, in addition to contaminated equipment, bed linens, or air droplets. The infection can originate from the outside environment, another infected patient, staff that may be infected, or in some cases, the source of the infection cannot be determined. In some cases the microorganism originates from the patient's own skin microbiota, becoming opportunistic after surgery or other procedures that compromise the protective skin barrier. Though the patient may have contracted the infection from their own skin, the infection is still considered nosocomial since it develops in the health care setting.[3] An easy way to understand the term is that the infection tends to lack evidence that it was incubating, or present when the patient entered the healthcare setting, thus meaning it was acquired post-admission.[3][4]
| Nosocomial infection | |
|---|---|
| Other names | HAI (Healthcare-Associated Infections) |
 | |
| Contaminated surfaces increase cross-transmission | |
| Specialty | Infectious disease |
In the United States, the Centers for Disease Control and Prevention estimated that roughly 1.7 million Healthcare-Associated infections, from all types of microorganisms, including bacteria and fungi combined, cause or contribute to 99,000 deaths each year.[5] In Europe, where hospital surveys have been conducted, the category of gram-negative infections are estimated to account for two-thirds of the 25,000 deaths each year.[6] Nosocomial infections can cause severe pneumonia and infections of the urinary tract, bloodstream and other parts of the body.[7][8] Many types display antimicrobial resistance, which can complicate treatment.
Types
Cause
Transmission
In-dwelling catheters have recently been identified with hospital acquired infections.[10] To deal with this complication procedures are used, called intravascular antimicrobial lock therapy that can reduce infections that are unexposed to blood-borne antibiotics.[11] Introducing antibiotics, including ethanol, into the catheter (without flushing it into the bloodstream) reduces the formation of biofilms.[9]
| Route | Description |
|---|---|
| Contact transmission | The most important and frequent mode of transmission of nosocomial infections is by direct contact. |
| Droplet transmission | Transmission occurs when droplets containing microbes from the infected person are propelled a short distance through the air and deposited on the patient's body; droplets are generated from the source person mainly by coughing, sneezing, and talking, and during the performance of certain procedures, such as bronchoscopy. |
| Airborne transmission | Dissemination can be either airborne droplet nuclei (small-particle residue {5 µm or smaller in size} of evaporated droplets containing microorganisms that remain suspended in the air for long periods of time) or dust particles containing the infectious agent. Microorganisms carried in this manner can be dispersed widely by air currents and may become inhaled by a susceptible host within the same room or over a longer distance from the source patient, depending on environmental factors; therefore, special air-handling and ventilation are required to prevent airborne transmission. Microorganisms transmitted by airborne transmission include Legionella, Mycobacterium tuberculosis and the rubeola and varicella viruses. |
| Common vehicle transmission | This applies to microorganisms transmitted to the host by contaminated items, such as food, water, medications, devices, and equipment. |
| Vector borne transmission | This occurs when vectors such as mosquitoes, flies, rats, and other vermin transmit microorganisms. |
Contact transmission is divided into two subgroups: direct-contact transmission and indirect-contact transmission.
| Route | Description |
|---|---|
| Direct-contact transmission | This involves a direct body surface-to-body surface contact and physical transfer of microorganisms between a susceptible host and an infected or colonized person, such as when a person turns a patient, gives a patient a bath, or performs other patient-care activities that require direct personal contact. Direct-contact transmission also can occur between two patients, with one serving as the source of the infectious microorganisms and the other as a susceptible host. |
| Indirect-contact transmission | This involves contact of a susceptible host with a contaminated intermediate object, usually inanimate, such as contaminated instruments, needles, or dressings, or contaminated gloves that are not changed between patients. In addition, the improper use of saline flush syringes, vials, and bags has been implicated in disease transmission in the US, even when healthcare workers had access to gloves, disposable needles, intravenous devices, and flushes.[12] |
Prevention

Controlling nosocomial infection is to implement QA/QC measures to the health care sectors, and evidence-based management can be a feasible approach. For those with ventilator-associated or hospital-acquired pneumonia, controlling and monitoring hospital indoor air quality needs to be on agenda in management,[13] whereas for nosocomial rotavirus infection, a hand hygiene protocol has to be enforced.[14][15][16]
To reduce the number of hospital-acquired infections, the state of Maryland implemented the Maryland Hospital-Acquired Conditions Program that provides financial rewards and penalties for individual hospitals. An adaptation of the Centers for Medicare & Medicaid Services payment policy causes poor-performing hospitals to lose up to 3% of their inpatient revenues, whereas hospitals that are able to decrease hospital-acquired infections can earn up to 3% in rewards. During the program's first two years, complication rates fell by 15.26% across all hospital-acquired conditions tracked by the state (including those not covered by the program), from a risk-adjusted complication rate of 2.38 per 1,000 people in 2009 to a rate of 2.02 in 2011. The 15.26% decline translates into more than $100 million in cost savings for the health care system in Maryland, with the largest savings coming from avoidance of urinary tract infections, sepsis and other severe infections, and pneumonia and other lung infections. If similar results could be achieved nationwide, the Medicare program would save an estimated $1.3 billion over two years, while the US health care system as a whole would save $5.3 billion.[17]
Sanitation
Hospitals have sanitation protocols regarding uniforms, equipment sterilization, washing, and other preventive measures. Thorough hand washing and/or use of alcohol rubs by all medical personnel before and after each patient contact is one of the most effective ways to combat nosocomial infections.[18] More careful use of antimicrobial agents, such as antibiotics, is also considered vital.[19] As many hospital-acquired infections caused by bacteria such as methicillin-resistant Staphylococcus aureus, methicillin-susceptible Staphylococcus aureus, and Clostridium difficile are caused by a breach of these protocols, it is common that affected patients make medical negligence claims against the hospital in question.[20]
Sanitizing surfaces is part of nosocomial infection in health care environments. Modern sanitizing methods such as Non-flammable Alcohol Vapor in Carbon Dioxide systems have been effective against gastroenteritis, methicillin-resistant Staphylococcus aureus, and influenza agents. Use of hydrogen peroxide vapor has been clinically proven to reduce infection rates and risk of acquisition. Hydrogen peroxide is effective against endospore-forming bacteria, such as Clostridium difficile, where alcohol has been shown to be ineffective.[21] Ultraviolet cleaning devices may also be used to disinfect the rooms of patients infected with Clostridium difficile or methicillin-resistant Staphylococcus aureus after discharge.[22]
Despite sanitation protocol, patients cannot be entirely isolated from infectious agents. Furthermore, patients are often prescribed antibiotics and other antimicrobial drugs to help treat illness; this may increase the selection pressure for the emergence of resistant strains.[23]
Sterilization
Sterilization goes further than just sanitizing. It kills all microorganisms on equipment and surfaces through exposure to chemicals, ionizing radiation, dry heat, or steam under pressure. Lately, steam sterilization of single-use implants have been questioned by US researchers who discovered contaminants and bacteria on single-use implants that have been repeated reprocessed in bulk before surgery. They suggested use of gamma-sterilization of implants, and providing implants in a single ready-to-use package to avoid repeated reprocessing of bulk implants for each surgery. The same concern was raised by Scottish Health Department more than a decade ago, and as a result Scottish hospitals underwent transition from steam sterilization of bulk implants to gamma sterilization of individually packaged implants. A petition has been filed by the reputable health science researcher Aakash Agarwal to ban steam sterilization of implants in US, requesting FDA to transition into a one-time gamma sterilization of single use implants.[24][25][26][27]
Isolation
Isolation is the implementation of isolating precautions designed to prevent transmission of microorganisms by common routes in hospitals. (See Universal precautions and Transmission-based precautions.) Because agent and host factors are more difficult to control, interruption of transfer of microorganisms is directed primarily at transmission for example isolation of infectious cases in special hospitals and isolation of patient with infected wounds in special rooms also isolation of joint transplantation patients on specific rooms.
Handwashing
Handwashing frequently is called the single most important measure to reduce the risks of transmitting skin microorganisms from one person to another or from one site to another on the same patient. Washing hands as promptly and thoroughly as possible between patient contacts and after contact with blood, body fluids, secretions, excretions, and equipment or articles contaminated by them is an important component of infection control and isolation precautions. The spread of nosocomial infections, among immunocompromised patients is connected with health care workers' hand contamination in almost 40% of cases, and is a challenging problem in the modern hospitals. The best way for workers to overcome this problem is conducting correct hand-hygiene procedures; this is why the WHO launched in 2005 the GLOBAL Patient Safety Challenge.[28] Two categories of micro-organisms can be present on health care workers' hands: transient flora and resident flora. The first is represented by the micro-organisms taken by workers from the environment, and the bacteria in it are capable of surviving on the human skin and sometimes to grow. The second group is represented by the permanent micro-organisms living on the skin surface (on the stratum corneum or immediately under it). They are capable of surviving on the human skin and to grow freely on it. They have low pathogenicity and infection rate, and they create a kind of protection from the colonization from other more pathogenic bacteria. The skin of workers is colonized by 3.9 x 104 – 4.6 x 106 cfu/cm2. The microbes comprising the resident flora are: Staphylococcus epidermidis, Staphylococcus hominis, and Microccocus, Propionibacterium, Corynebacterium, Dermobacterium, and Pitosporum spp., while transient organisms are Staphylococcus aureus, and Klebsiella pneumoniae, and Acinetobacter, Enterobacter and Candida spp. The goal of hand hygiene is to eliminate the transient flora with a careful and proper performance of hand washing, using different kinds of soap, (normal and antiseptic), and alcohol-based gels. The main problems found in the practice of hand hygiene is connected with the lack of available sinks and time-consuming performance of hand washing. An easy way to resolve this problem could be the use of alcohol-based hand rubs, because of faster application compared to correct hand-washing.[29]
Improving patient hand washing has also been shown to reduce the rate of nosocomial infection. Patients who are bed-bound often do not have as much access to clean their hands at mealtimes or after touching surfaces or handling waste such as tissues. By reinforcing the importance of handwashing and providing sanitizing gel or wipes within reach of the bed, nurses were directly able to reduce infection rates. A study published in 2017 demonstrated this by improving patient education on both proper hand-washing procedure and important times to use sanitizer and successfully reduced the rate of enterococci and Staphylococcus aureus.[30]
All visitors must follow the same procedures as hospital staff to adequately control the spread of infections. Moreover, multidrug-resistant infections can leave the hospital and become part of the community flora if steps are not taken to stop this transmission.
It is unclear whether or not nail polish or rings affected surgical wound infection rates.[31]
Gloves
In addition to hand washing, gloves play an important role in reducing the risks of transmission of microorganisms. Gloves are worn for three important reasons in hospitals. First, they are worn to provide a protective barrier for personnel, preventing large scale contamination of the hands when touching blood, body fluids, secretions, excretions, mucous membranes, and non-intact skin. In the United States, the Occupational Safety and Health Administration has mandated wearing gloves to reduce the risk of bloodborne pathogen infections.[32] Second, gloves are worn to reduce the likelihood that microorganisms present on the hands of personnel will be transmitted to patients during invasive or other patient-care procedures that involve touching a patient's mucous membranes and nonintact skin. Third, they are worn to reduce the likelihood that the hands of personnel contaminated with micro-organisms from a patient or a fomite can transmit those micro-organisms to another patient. In this situation, gloves must be changed between patient contacts, and hands should be washed after gloves are removed.
Wearing gloves does not replace the need for handwashing due to the possibility of contamination when gloves are replaced, or by damage to the glove. Doctors wearing the same gloves for multiple patient operations presents an infection control hazard.[33] Furthermore, gloves get contaminated during surgical site preparation and the latest multicenter clinical trial led by Aakash Agarwal and his team of researchers have shown that implants should be guarded inside the sterile field to avoid direct contact between the gloves and the implantable device. This method of guarding implants intraoperatively avoids transfer of bacteria from the gloves, air, and contaminated surfaces to the patients (through the implants as vectors).[34][35][36][37][38]
Antimicrobial surfaces
Micro-organisms are known to survive on inanimate ‘touch’ surfaces for extended periods of time.[39] This can be especially troublesome in hospital environments where patients with immunodeficiencies are at enhanced risk for contracting nosocomial infections.
Touch surfaces commonly found in hospital rooms, such as bed rails, call buttons, touch plates, chairs, door handles, light switches, grab rails, intravenous poles, dispensers (alcohol gel, paper towel, soap), dressing trolleys, and counter and table tops are known to be contaminated with Staphylococcus, methicillin-resistant Staphylococcus aureus (one of the most virulent strains of antibiotic-resistant bacteria) and vancomycin-resistant Enterococcus.[40] Objects in closest proximity to patients have the highest levels of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. This is why touch surfaces in hospital rooms can serve as sources, or reservoirs, for the spread of bacteria from the hands of healthcare workers and visitors to patients.
A number of compounds can decrease the risk of bacteria growing on surfaces including: copper, silver, and germicides.[41]
There have been a number of studies evaluating the use of no-touch cleaning systems particularly the use of ultraviolet C devices. One review was inconclusive due to lack of, or of poor quality evidence.[42] Other reviews have found some evidence, and growing evidence of their effectiveness.[43][44]
Treatment
Two of the bacteria species most likely to infect patients are the Gram-positive strains of methicillin-resistant Staphylococcus aureus, and Gram-negative Acinetobacter baumannii. While antibiotic drugs to treat diseases caused by methicillin-resistant Staphylococcus aureus are available, few effective drugs are available for Acinetobacter. Acinetobacter bacteria are evolving and becoming immune to antibiotics, so in many cases, polymyxin-type antibacterials need to be used. "In many respects it’s far worse than MRSA," said a specialist at Case Western Reserve University.[45]
Another growing disease, especially prevalent in New York City hospitals, is the drug-resistant, Gram-negative Klebsiella pneumoniae. An estimated more than 20% of the Klebsiella infections in Brooklyn hospitals "are now resistant to virtually all modern antibiotics, and those supergerms are now spreading worldwide."[45]
The bacteria, classified as Gram-negative because of their color on the Gram stain, can cause severe pneumonia and infections of the urinary tract, bloodstream, and other parts of the body. Their cell structures make them more difficult to attack with antibiotics than Gram-positive organisms like methicillin-resistant Staphylococcus aureus. In some cases, antibiotic resistance is spreading to Gram-negative bacteria that can infect people outside the hospital. "For gram-positives we need better drugs; for gram-negatives we need any drugs," said Dr. Brad Spellberg, an infectious-disease specialist at Harbor-UCLA Medical Center, and the author of Rising Plague, a book about drug-resistant pathogens.[45]
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection and accounts for approximately one-fourth of all infections in the intensive care unit (ICU).[46] HAP, or nosocomial pneumonia, is a lower respiratory infection that was not incubating at the time of hospital admission and that presents clinically 2 or more days after hospitalization.[47] Ventilator-associated pneumonia (VAP) is defined as HAP in patients receiving mechanical ventilation. The incidence of VAP is 10%-30% among patients who require mechanical ventilation for >48 h.[48] A standard treatment protocol is based on accurate diagnosis definitions, microbiological confirmation of VAP, and the administration of imipenem plus ciprofloxacin as initial empirical antibiotic treatment.[49]
One-third of nosocomial infections are considered preventable. The CDC estimates 2 million people in the United States are infected annually by hospital-acquired infections, resulting in 99,000 deaths.[50] The most common nosocomial infections are of the urinary tract, surgical site and various pneumonias.[51]
An alternative treatment targeting localised infections is the use of irradiation by ultraviolet C.[52]
Epidemiology
The methods used differ from country to country (definitions used, type of nosocomial infections covered, health units surveyed, inclusion or exclusion of imported infections, etc.), so the international comparisons of nosocomial infection rates should be made with the utmost care.
Belgium
In Belgium the prevalence of nosocomial infections is about 6.2%. Annually about 125,500 patients become infected by a nosocomial infection, resulting in almost 3000 deaths. The extra costs for the health insurance are estimated to be approximately €400 million/year.[53]
France
Estimates ranged from 6.7% in 1990 to 7.4% (patients may have several infections).[54] At national level, prevalence among patients in health care facilities was 6.7% in 1996,[55] 5.9% in 2001[56] and 5.0% in 2006.[57] The rates for nosocomial infections were 7.6% in 1996, 6.4% in 2001 and 5.4% in 2006.
In 2006, the most common infection sites were urinary tract infections (30,3%), pneumopathy (14,7%), infections of surgery site (14,2%). Infections of the skin and mucous membrane (10,2%), other respiratory infections (6,8%) and bacterial infections / blood poisoning (6,4%).[58] The rates among adult patients in intensive care were 13,5% in 2004, 14,6% in 2005, 14,1% in 2006 and 14.4% in 2007.[59]
Nosocomial infections are estimated to make patients stay in the hospital four to five additional days. Around 2004–2005, about 9,000 people died each year with a nosocomial infection, of which about 4,200 would have survived without this infection.[60]
Finland
Rate were estimated at 8.5% of patients in 2005.[61]
Italy
Since 2000, estimates show about a 6.7% infection rate, i.e. between 450,000 and 700,000 patients, which caused between 4,500 and 7,000 deaths.[62] A survey in Lombardy gave a rate of 4.9% of patients in 2000.[63]
Switzerland
Estimates range between 2 and 14%.[64] A national survey gave a rate of 7.2% in 2004.[65]
United Kingdom
In 2012 the Health Protection Agency reported the prevalence rate of hospital-acquired infectionIs in England was 6.4% in 2011, against a rate of 8.2% in 2006.[66] with respiratory tract, urinary tract and surgical site infections the most common types of infections reported.[66] In 2018 it was reported that in-hospital infections had risen from 5,972 in 2008 to 48,815 in 2017.[67]
United States
The Centers for Disease Control and Prevention (CDC) estimated roughly 1.7 million hospital-associated infections, from all types of bacteria combined, cause or contribute to 99,000 deaths each year.[68] Other estimates indicate 10%, or 2 million, patients a year become infected, with the annual cost ranging from $4.5 billion to $11 billion.[69] In the US, the most frequent type of infection hospital is urinary tract infection (36%), followed by surgical site infection (20%), and bloodstream infection and pneumonia (both 11%).[45]
History
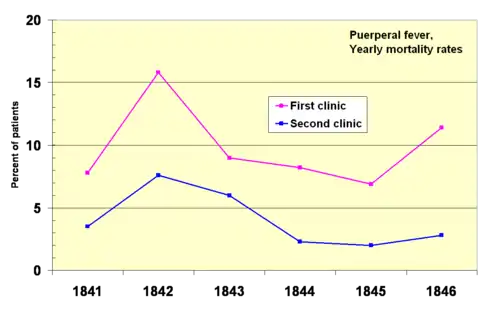
In 1841, Ignaz Semmelweis, a Hungarian obstetrician was working at a Vienna maternity hospital. He was "shocked" by the death rate of women who developed puerperal fever. He documented that mortality was three times higher in the ward where the medical students were delivering babies than in the next ward that was staffed by midwifery students.[70] The medical students were also routinely working with cadavers. He compared the rates of infection with a similar hospital in Dublin, Ireland and hypothesized that it was the medical students who somehow were infecting the women after labor. He instituted mandatory hand-washing in May 1847 and infection rates dropped dramatically. Louis Pasteur proposed the germ theory of disease and began his work on cholera in 1865 by identifying that it was microorganisms that were associated with disease.[71][72]
See also
References
- Rosenthal VD, et al. (2012). International Nosocomial Infection Control Consortium report, data summary of 36 countries, for 2004-2009. In: American Journal of Infection Control; 40(5):396-407. https://doi.org/10.1016/j.ajic.2011.05.020
- "HAI Data and Statistics". cdc.gov. 2018-01-10. Retrieved 2018-01-13.
- Monegro, Alberto F.; Muppidi, Vijayadershan; Regunath, Hariharan (2020), "Hospital Acquired Infections", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 28722887, retrieved 2021-02-01
- Sydnor, Emily R. M.; Perl, Trish M. (January 2011). "Hospital epidemiology and infection control in acute-care settings". Clinical Microbiology Reviews. 24 (1): 141–173. doi:10.1128/CMR.00027-10. ISSN 1098-6618. PMC 3021207. PMID 21233510.
- Klevens, R. Monina; Edwards, Jonathan R.; Richards, Chesley L.; Horan, Teresa C.; Gaynes, Robert P; Pollock, Daniel A.; Cardo, Denise M. (2007). "Estimating Healthcare-associated Infections and Deaths in U.S. Hospitals, 2002". Public Health Reports. 122 (2): 160–166. doi:10.1177/003335490712200205. PMC 1820440. PMID 17357358.
- Pollack, Andrew (2010-02-26). "Doctors Struggle to Treat Gram-Negative Bacterial Infections". The New York Times. ISSN 0362-4331. Retrieved 2019-11-15.
- Burke A Cunha (July 30, 2018). John L Brusch (ed.). "Hospital-Acquired Pneumonia (Nosocomial Pneumonia) and Ventilator-Associated Pneumonia: Overview, Pathophysiology, Etiology". Webscape.
- Su, Lin-Hui; Ou, Jonathan T.; Leu, Hsieh-Shong; Chiang, Ping-Cherng; Chiu, Yueh-Pi; Chia, Ju-Hsin; Kuo, An-Jing; Chiu, Cheng-Hsun; Chu, Chishih (2003-10-01). "Extended Epidemic of Nosocomial Urinary Tract Infections Caused by Serratia marcescens". Journal of Clinical Microbiology. 41 (10): 4726–4732. doi:10.1128/JCM.41.10.4726-4732.2003. ISSN 0095-1137. PMC 254321. PMID 14532211.
- Akbari, Freshta; Kjellerup, Birthe (2015). "Elimination of Bloodstream Infections Associated with Candida albicans Biofilm in Intravascular Catheters". Pathogens. 4 (3): 457–469. doi:10.3390/pathogens4030457. ISSN 2076-0817. PMC 4584267. PMID 26131615.
- "Catheter-associated Urinary Tract Infections (CAUTI)". cdc.gov. 2017-07-19. Retrieved 2018-01-13.
- Justo, JA; Bookstaver, PB (2014). "Antibiotic lock therapy: review of technique and logistical challenges". Infection and Drug Resistance. 7: 343–63. doi:10.2147/IDR.S51388. PMC 4271721. PMID 25548523.
- Jain SK, Persaud D, Perl TM, et al. (July 2005). "Nosocomial malaria and saline flush". Emerging Infect. Dis. 11 (7): 1097–9. doi:10.3201/eid1107.050092. PMC 3371795. PMID 16022788.
- Leung M, Chan AH (March 2006). "Control and management of hospital indoor air quality". Med. Sci. Monit. 12 (3): SR17–23. PMID 16501436.
- Chan PC, Huang LM, Lin HC, et al. (April 2007). "Control of an outbreak of pandrug-resistant Acinetobacter baumannii colonization and infection in a neonatal intensive care unit". Infect Control Hosp Epidemiol. 28 (4): 423–9. doi:10.1086/513120. PMID 17385148.
- Traub-Dargatz JL, Weese JS, Rousseau JD, Dunowska M, Morley PS, Dargatz DA (July 2006). "Pilot study to evaluate 3 hygiene protocols on the reduction of bacterial load on the hands of veterinary staff performing routine equine physical examinations". Can. Vet. J. 47 (7): 671–6. PMC 1482439. PMID 16898109.
- Katz JD (September 2004). "Hand washing and hand disinfection: more than your mother taught you". Anesthesiol Clin North America. 22 (3): 457–71, vi. doi:10.1016/j.atc.2004.04.002. PMID 15325713.
- "Statewide, All-Payer Financial Incentives Significantly Reduce Hospital-Acquired Conditions in Maryland Hospitals". Agency for Healthcare Research and Quality. 2013-07-03. Retrieved 2013-07-06.
- McBryde ES, Bradley LC, Whitby M, McElwain DL (October 2004). "An investigation of contact transmission of methicillin-resistant Staphylococcus aureus". Journal of Hospital Infection. 58 (2): 104–8. doi:10.1016/j.jhin.2004.06.010. PMID 15474180.
- Lautenbach E (2001). "Chapter 14. Impact of Changes in Antibiotic Use Practices on Nosocomial Infections and Antimicrobial Resistance—Clostridium difficile and Vancomycin-resistant Enterococcus (VRE)". In Markowitz AJ (ed.). Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Agency for Healthcare Research and Quality.
- "Hospital Negligence Claims". PatientClaimLine.com. Retrieved 2019-08-21.
- Otter JA, French GL (January 2009). "Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor". J. Clin. Microbiol. 47 (1): 205–7. doi:10.1128/JCM.02004-08. PMC 2620839. PMID 18971364.
- "Performance Feedback, Ultraviolet Cleaning Device, and Dedicated Housekeeping Team Significantly Improve Room Cleaning, Reduce Potential for Spread of Common, Dangerous Infection". Agency for Healthcare Research and Quality. 2014-01-15. Retrieved 2014-01-20.
- Kolár, M.; Urbánek, K.; Látal, T. (May 2001). "Antibiotic selective pressure and development of bacterial resistance". International Journal of Antimicrobial Agents. 17 (5): 357–363. doi:10.1016/S0924-8579(01)00317-X. ISSN 0924-8579. PMID 11337221.
- "The Different Methods of Sterilizing Medical Equipment". Gibraltar Laboratories. 2013-05-31. Retrieved 2018-12-06.
- "11 Investigates: Surgical implants raising contamination concerns". wtol.com. Retrieved 2020-07-28.
- "Ban 'Reprocessing' of Spinal Surgery Screws, Experts Say". Medscape. Retrieved 2020-07-28.
- Hudson, Jocelyn (2019-01-16). "Banned in the USA: Petition calls for FDA to prohibit reprocessed pedicle screws". Spinal News International. Retrieved 2020-07-28.
- World Alliance for patient safety. WHO Guidelines on Hand Hygiene in Health Care. http://www.who.int/rpc/guidelines/9789241597906/en/. 2009
- Hugonnet S, Perneger TV, Pittet D. Alcohol based hand rub improves compliance with hand hygiene in intensive care units. Arch Intern med 2002; 162: 1037-1043.
- Haverstick, Stacy; Goodrich, Cara; Freeman, Regi; James, Shandra; Kullar, Rajkiran; Ahrens, Melissa (June 2017). "Patients' Hand Washing and Reducing Hospital-Acquired Infection". Critical Care Nurse. 37 (3): e1–e8. doi:10.4037/ccn2017694. PMID 28572111.
- Arrowsmith, VA; Taylor, R (Aug 4, 2014). "Removal of nail polish and finger rings to prevent surgical infection". The Cochrane Database of Systematic Reviews (8): CD003325. doi:10.1002/14651858.CD003325.pub3. PMC 7163182. PMID 25089848.
- "Occupational Exposure to Bloodborne Pathogens;Needlestick and Other Sharps Injuries; Final Rule. - 66:5317-5325". Osha.gov. Retrieved 2011-07-11.
- Osterweil, Neil (June 19, 2016). "Bacteria Can Persist on Gloves, Transfer to Surfaces". Medscape.
- "Pedicle screw handling techniques "lead to contamination of screws"". Spinal News International. 2020-06-05. Retrieved 2020-07-29.
- Marshall, Suzie (2018-09-14). "Surgeons call for two-step asepsis process & a ban on pedicle screw reuse". Spinal News International. Retrieved 2020-07-29.
- Agarwal, Aakash; PhD; An, Neel; MD; Wang, Jeffrey C.; MD. "Reprocessing of Pedicle Screws and Exposure in Sterile-Field Leads to Infection in Spinal Surgery". SpineUniverse. Retrieved 2020-07-29.
- "The Hardest Decision Any Spine Surgeon Has to Make | Orthopedics This Week - Part 2". ryortho.com. Retrieved 2020-07-29.
- "Are Your Sterile Implants Really Sterile? | Orthopedics This Week". ryortho.com. Retrieved 2020-07-29.
- Wilks, S.A., Michels, H., Keevil, C.W., 2005, The Survival of Escherichia Coli O157 on a Range of Metal Surfaces, International Journal of Food Microbiology, Vol. 105, pp. 445–454. and Michels, H.T. (2006), Anti-Microbial Characteristics of Copper, ASTM Standardization News, October, pp. 28-31
- U.S. Department of Defense-funded clinical trials, as presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy in Washington, D.C., October 28, 2008
- Weber, DJ; Rutala, WA (May 2013). "Self-disinfecting surfaces: review of current methodologies and future prospects". American Journal of Infection Control. 41 (5 Suppl): S31-5. doi:10.1016/j.ajic.2012.12.005. PMID 23622745.
- "Portable Ultraviolet Light Surface-Disinfecting Devices for Prevention of Hospital-Acquired Infections: A Health Technology Assessment". Ontario Health Technology Assessment Series. 18 (1): 1–73. 2018. PMC 5824029. PMID 29487629.
- Weber, DJ; Kanamori, H; Rutala, WA (August 2016). "'No touch' technologies for environmental decontamination: focus on ultraviolet devices and hydrogen peroxide systems". Current Opinion in Infectious Diseases. 29 (4): 424–31. doi:10.1097/QCO.0000000000000284. PMID 27257798.
- Weber, DJ; Rutala, WA; Anderson, DJ; Chen, LF; Sickbert-Bennett, EE; Boyce, JM (2 May 2016). "Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials". American Journal of Infection Control. 44 (5 Suppl): e77-84. doi:10.1016/j.ajic.2015.11.015. PMC 7132689. PMID 27131140.
- Pollack, Andrew. "Rising Threat of Infections Unfazed by Antibiotics" New York Times, Feb. 27, 2010
- Antoni Torres, Miquel Ferrer, Joan Ramón Badia, Treatment Guidelines and Outcomes of Hospital-Acquired and Ventilator-Associated Pneumonia, Clinical Infectious Diseases, Volume 51, Issue Supplement_1, January–February 1988, Pages S48–S53, https://doi.org/10.1086/653049
- https://emedicine.medscape.com/article/234753-overview
- Torres A, Ewig S, Lode H, Carlet J. Defining, treating and preventing hospital acquired pneumonia: European perspective, Intensive Care Med, 2009, vol. 35 (pg. 9-29)
- Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia, Crit Care Med, 2001, vol. 29 (pg. 1109-1115)
- "99,000 Americans die of Healthcare-Acquired Infections Every Year". 2013-07-26.
- Klevens RM, Edwards JR, Richards CL, et al. (2007). "Estimating health care-associated infections and deaths in U.S. hospitals, 2002". Public Health Rep. 122 (2): 160–6. doi:10.1177/003335490712200205. PMC 1820440. PMID 17357358.
- Dai, T; Vrahas, MS; Murray, CK; Hamblin, MR (February 2012). "Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections?". Expert Review of Anti-infective Therapy. 10 (2): 185–95. doi:10.1586/eri.11.166. PMC 3292282. PMID 22339192.
- Federaal Kenniscentrum voor de Gezondheidszorg (2009) Nosocomiale Infecties in België, deel II: Impact op Mortaliteit en Kosten. KCE-rapport 102A.
- Quenon JL, Gottot S, Duneton P, Lariven S, Carlet J, Régnier B, Brücker G. Enquête nationale de prévalence des infections nosocomiales en France : Hôpital Propre (octobre 1990). BEH n° 39/1993.
- Comité technique des infections nosocomiales (CTIN), Cellule infections nosocomiales, CClin Est, CClin Ouest, CClin Paris-Nord, CClin Sud-Est, CClin Sud-Ouest, avec la participation de 830 établissements de santé. Enquête nationale de prévalence des infections nosocomiales,1996, BEH n° 36/1997, 2 sept. 1997, 4 pp.. Résumé.
- Lepoutre A, Branger B, Garreau N, Boulétreau A, Ayzac L, Carbonne A, Maugat S, Gayet S, Hommel C, Parneix P, Tran B pour le Réseau d’alerte, d’investigation et de surveillance des infections nosocomiales (Raisin). Deuxième enquête nationale de prévalence des infections nosocomiales, France, 2001, Surveillance nationale des maladies infectieuses, 2001-2003. Institut de veille sanitaire, sept. 2005, 11 pp. Résumé.
- Institut de veille sanitaire Enquête nationale de prévalence des infections nosocomiales, France, juin 2006, Volume 1 – Méthodes, résultats, perspectives, mars 2009, ii + 81 pp. Volume 2 – Annexes, mars 2009, ii + 91 pp. Synthèse des résultats, Mars 2009, 11 pp.
- Institut de veille sanitaire Enquête nationale de prévalence des infections nosocomiales, France, juin 2006, Vol. 1, Tableau 31, p. 24.
- Réseau REA-Raisin « Surveillance des infections nosocomiales en réanimation adulte. France, résultats 2007 », Institut de veille sanitaire, Sept. 2009, ii + 60 pp.
- Vasselle, Alain « Rapport sur la politique de lutte contre les infections nosocomiales », Office parlementaire d'évaluation des politiques de santé, juin 2006, 290 pp. (III.5. Quelle est l’estimation de la mortalité attribuable aux IN ?).
- Lyytikainen O, Kanerva M, Agthe N, Mottonen T and the Finish Prevalence Survey Study Group. National Prevalence Survey on Nosocomial Infections in Finnish Acute Care Hospitals, 2005. 10th Epiet Scientific Seminar. Mahon, Menorca, Spain, 13–15 October 2005 [Poster].
- L'Italie scandalisée par "l'hôpital de l'horreur", Éric Jozsef, Libération, January 17, 2007 (in French)
- Liziolia A, Privitera G, Alliata E, Antonietta Banfi EM, Boselli L, Panceri ML, Perna MC, Porretta AD, Santini MG, Carreri V. Prevalence of nosocomial infections in Italy: result from the Lombardy survey in 2000. J Hosp Infect 2003;54:141-8.
- "Facts sheet - Swiss Hand Hygiene Campaign" (in French). Archived from the original (.doc) on 2007-09-30.
- Sax H, Pittet D (2005). "Résultats de l'enquête nationale de prévalence des infections nosocomiales de 2004 (snip04)". Swiss-NOSO (in French). 12 (1): 1–4. Archived from the original on November 11, 2007.
- "English National Point Prevalence Survey on Healthcare-associated Infections and Antimicrobial Use, 2011" (PDF). Health Protection Agency. Archived from the original (PDF) on 8 December 2015. Retrieved 28 November 2015.
- "Deadly hospital infections quadruple as staff struggle to fight superbugs". Daily Mirror. 24 October 2018. Retrieved 2 December 2018.
- Klevens, R Monina et al. "Estimating Health Care-associated Infections and Deaths in U.S. Hospitals, 2002." Public Health Reports 122.2 (2007): 160–166.
- Hospital-acquired infection (HAI) diagnostics market is forecasted to reach $4,386.6 million by 2023, growing at a CAGR of 7.6% during 2017–2023, P&S Intelligence
- Kadar N (January 2019). "Rediscovering Ignaz Philipp Semmelweis (1818-1865)". Am. J. Obstet. Gynecol. 220 (1): 26–39. doi:10.1016/j.ajog.2018.11.1084. PMID 30444981.
- Pommerville, Jeffrey (2014). Fundamentals of microbiology. Burlington, MA: Jones & Bartlett Learning. ISBN 9781449647964.
- Wyklicky, H.; Skopec, M. (Sep–Oct 1983). "Ignaz Philipp Semmelweis, the prophet of bacteriology". Infect Control. 4 (5): 367–70. doi:10.1017/S0195941700059762. PMID 6354955. Archived from the original on 2008-04-04. Retrieved 2015-11-28.
External links
| Classification | |
|---|---|
| External resources |
 The dictionary definition of nosocomial at Wiktionary
The dictionary definition of nosocomial at Wiktionary