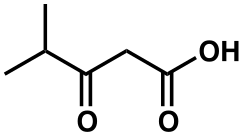
beta-Ketoisocaproic acid
β-Ketoisocaproic acid is an intermediate in the metabolism of leucine.[2][3] Its metabolic precursor and metabolic product in the leucine metabolic pathway are β-leucine and β-ketoisocaproyl-CoA, respectively.[2]
 | |
| Names | |
|---|---|
| IUPAC name
4-methyl-3-oxopentanoic acid | |
| Systematic IUPAC name
4-methyl-3-oxopentanoic acid[1] | |
| Other names
4-Methyl-2-oxovaleric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | Beta-ketoisocaproic+acid |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.143 g·mol−1 |
| Density | 1.1 g cm−3 (at 20 °C) |
| Boiling point | 236 °C (457 °F; 509 K) ±23 at 760 mmHg |
| log P | 0.36 |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R34 |
| S-phrases (outdated) | S26, S36/37/39, S45 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- CID 440024 from PubChem
- Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds ...
Figure 8.57: Metabolism of L-LEUCINE - "Leucine metabolism". BRENDA. Technische Universität Braunschweig. Archived from the original on 17 August 2016. Retrieved 12 August 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.