Caffeine citrate
Caffeine citrate, sold under the brand name Cafcit among others, is a medication used to treat a lack of breathing in premature babies.[3] Specifically it is given to babies who are born at less than 35 weeks or weigh less than 2 kilograms (4.4 lb) once other causes are ruled out.[4] It is given by mouth or slow injection into a vein.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Cafcit, Gencebok, Cafnea, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, intravenous (IV) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.472 |
| Chemical and physical data | |
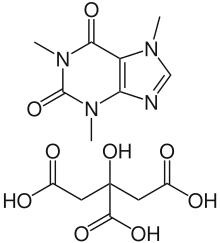
| Formula | C14H18N4O9 |
| Molar mass | 386.317 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects can include problems feeding, increased heart rate, low blood sugar, necrotizing enterocolitis, and kidney problems.[4][3] Testing blood caffeine levels is occasionally recommended.[3] It is a citric acid salt of caffeine.[5] Caffeine citrate is in the xanthine family of medication.[4] It works by stimulating the respiratory centers in the brain.[3]
Caffeine was discovered in 1819.[6] It is on the World Health Organization's List of Essential Medicines.[7] The intravenous form may also be taken by mouth.[8]
In June 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of Gencebok.[9] It was approved for use in the European Union in August 2020.[2]
Medical uses
Caffeine citrate is generally the preferred treatment for apnea of prematurity.[4] It has fewer side effects as compared to theophylline.[4]
Caffeine improves airway function in asthma, increasing forced expiratory volume (FEV1) by 5% to 18%, with this effect lasting for up to four hours.[10]
Mechanism
In method of action, the preparation is exactly identical to that of caffeine base as the citrate counter ion dissociates in water. Doses of caffeine citrate, due to the added weight of the citrate moiety, are understandably higher than with caffeine base, i.e., it takes a larger dose to get the same amount of caffeine.[8] The ratio of therapeutic doses of caffeine base to its citrate salt is typically 1:2.[8] Dosing should therefore be clearly distinguished.[8]
Manufacture
The drug is prepared by combining anhydrous caffeine with citric acid monohydrate and sodium citrate dihydrate.
References
- "Cafcit- caffeine citrate injection". DailyMed. 3 January 2020. Retrieved 27 August 2020.
- "Gencebok EPAR". European Medicines Agency (EMA). 19 June 2020. Retrieved 27 August 2020.
- "Caffeine; Caffeine and Sodium Benzoate Injection; Caffeine Citrate". The American Society of Health-System Pharmacists. Archived from the original on 16 July 2017. Retrieved 8 December 2016.
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 485. hdl:10665/44053. ISBN 9789241547659.
- Donn, Steven M.; Sinha, Sunil K. (2012). Manual of Neonatal Respiratory Care. Springer Science & Business Media. p. 457. ISBN 9781461421559. Archived from the original on 2016-12-30.
- Brown, Nathan (2015). In Silico Medicinal Chemistry: Computational Methods to Support Drug Design. Royal Society of Chemistry. p. 20. ISBN 9781782621638. Archived from the original on 2016-12-29.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Ainsworth, Sean B. (2014). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (7 ed.). John Wiley & Sons. p. 120. ISBN 9781118819517. Archived from the original on 2016-12-30.
- "Gencebok: Pending EC decision". European Medicines Agency (EMA). 25 June 2020. Retrieved 26 June 2020.
- Welsh EJ, Bara A, Barley E, Cates CJ (January 2010). "Caffeine for asthma" (PDF). Cochrane Database Syst Rev (1): CD001112. doi:10.1002/14651858.CD001112.pub2. PMC 7053252. PMID 20091514.
External links
- "Caffeine citrate". Drug Information Portal. U.S. National Library of Medicine.
- "Caffeine Citrate (CUI C0054436)". NCI Metathesaurus.
