Chrysophanol
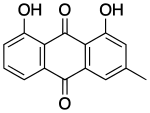
Chrysophanol, also known as chrysophanic acid, is a fungal isolate and a natural anthraquinone.
 | |
| Names | |
|---|---|
| IUPAC name
1,8-Dihydroxy-3-methyl-9,10-anthraquinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.885 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10O4 | |
| Molar mass | 254.241 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of action
Chrysophanol blocks the proliferation of colon cancer cells in vitro.[1] It induces the necrosis of cells via a reduction in ATP levels.[2] Chrysophanol attenuates the effects of lead exposure in mice by reducing hippocampal neuronal cytoplasmic edema, enhancing mitochondrial crista fusion, significantly increasing memory and learning abilities, reducing lead content in blood, heart, brain, spleen, kidney and liver, promoting superoxide dismutase and glutathione peroxidase activities and reducing malondialdehyde level in the brain, kidney and liver.[3]
References
- Lee, MS; Cha, EY; Sul, JY; Song, IS; Kim, JY (2011). "Chrysophanic acid blocks proliferation of colon cancer cells by inhibiting EGFR/mTOR pathway". Phytotherapy Research. 25 (6): 833–7. doi:10.1002/ptr.3323. PMID 21089180.
- Burnstock, G; Di Virgilio, F (Dec 2013). "Purinergic signalling and cancer". Purinergic Signalling. 9 (4): 491–540. doi:10.1007/s11302-013-9372-5. PMC 3889385. PMID 23797685.
- Zhang, J; Yan, C; Wang, S; Hou, Y; Xue, G; Zhang, L (2014). "Chrysophanol attenuates lead exposure-induced injury to hippocampal neurons in neonatal mice". Neural Regeneration Research. 9 (9): 924–30. doi:10.4103/1673-5374.133141. PMC 4146226. PMID 25206913.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.