Choristodera
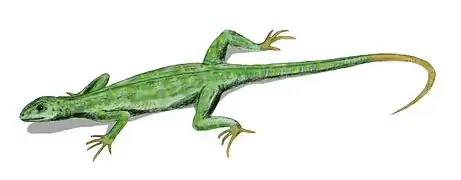
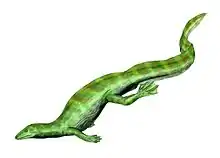
Choristodera is an extinct order of semiaquatic diapsid reptiles that ranged from the Middle Jurassic, or possibly Triassic, to the late Miocene (168 to 11 million years ago). Choristoderes are morphologically diverse, with the best known members being the gharial-like neochoristoderes such as Champsosaurus. Other choristoderans had lizard-like or long necked morphologies. Choristoderes appear to have been confined to the Northern Hemisphere, having been found in North America, Asia, and Europe, and possibly also North Africa.[1] Cladists have placed choristoderes as neodiapsids, but the exact phylogenetic position of Choristodera is still uncertain. It has been proposed that they may represent basal lepidosauromorphs or archosauromorphs.
| Choristodera | |
|---|---|
 | |
| Skeleton of Philydrosaurus | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Clade: | Sauria |
| Order: | †Choristodera Cope, 1876 |
| Subgroups | |
| |
History of Discovery

Choristodera was erected in 1876, originally as a suborder of Rhynchocephalia by Edward Drinker Cope to contain Champsosaurus, which was described from Late Cretaceous strata of Montana by Cope in the same paper.[2][3] A year later, Simoedosaurus was described by Paul Gervais from Upper Paleocene deposits at Cernay, near Rheims, France. These remained the only described choristoderes for over a century, until new taxa were described in the late 20th century.[4] Beginning in the late 1970s, additional taxa were described by Soviet-Mongolian teams from Lower Cretaceous sediments of Mongolia. In studies from 1989 to 1991, Susan E. Evans described new material of Cteniogenys from the Middle Jurassic of Britain. The genus had been first been described by Charles W. Gilmore in 1928 from the Late Jurassic of the western United States, and had previously been enigmatic. The studies revealed it to be a small bodied choristodere, different from the crocodile-like forms previously known.[5]
Description
_NMNS.jpg.webp)
Neochoristoderes such as Champsosaurus are the best-known group of the Choristodera. They resembled modern gharials (gavials) or false gharials. The skull of these animals have a long, thin snout filled with small, sharp conical teeth. Other choristoderes belong to the paraphyletic assemblage of "non-neochoristoderes", which are mostly small lizard like forms, though Shokawa, Khurendukhosaurus and Hyphalosaurus possess long plesiosaur like necks.[4]
Skeletal anatomy
According Matsumoto and colleagues (2019), choristoderes are united by the presence of 9 synapomorphies, including a median contact of the elongated prefrontal bones separating the nasal bones from the frontal bones, the dorsal flange of the maxilla is inflected medially, the parietal foramen are absent, the squamosal bones are expanded posterior to the occipital condyle, the teeth are conical and sub-thecodont, the dentaries are slender with elongated grooves running along the labial surface of the bone, additional sacral vertebrae are present, expanded "spine tables" are present on the vertebrae, and the surfaces of both ends of vertebral centra are flat (amphiplatyan).[6] All known choristoderans possess or are inferred to possess a novel skull ossification referred to as the "neomorphic bone", which is small in primitive members of the group, suggesting it originated via a neutral mutation.[7] Choristoderes also possess gastralia (rib-like bones situated in abdomen), which were inherited from the neodiapsid ancestor.[8]
Dentition
Most choristoderes have rather simple undifferentiated (homodont) teeth, with striated enamel covering the tooth crown but not the base. Neochoristoderes have teeth completely enveloped in striated enamel with an enamel infolding at the base, labiolingually compressed and hooked, the exception being Ikechosaurus which has still rather simple teeth aside from the start of an enamel infolding. There is some tooth differentiation among neochoristoderes, with the anterior teeth being sharper and more slender than posterior teeth. Choristoderes retain palatal teeth (teeth present in the roof of the mouth). Unlike most diapsid groups, where palatal teeth are reduced or lost completely, the palatal teeth in choristoderes are extensively developed indicating food manipulation in the mouth, probably in combination with the tongue.[9]
Skin
An exceptionally preserved specimen of Monjurosuchus preserves pleated skin, which indicates that in life it was probably thin and soft. The preserved scales are small and overlapping, and are smaller on the ventral underside of the body than the dorsal surface. A double row of larger ovoid scales runs along the dorsum (upper midline) of the body. The fossil also preserves webbed feet.[10] Hyphalosaurus was covered mostly in small, irregularly patterned polygonal scales, though these varied across the body. The scales of the hind legs were smaller, finer and more irregular than those of the torso, while the scales of the tail were nearly square and arranged in more regular rows. In addition to the small scales, two rows of large, round scutes with shallow keels ran along the animals sides. One row ran directly along the flank, with the other either slightly higher or lower and composed of scutes only 1/4 the size of the flank scutes. The flank row of larger scutes extended all the way to the base of the tail, and remained uniform in size across the entire row. The tail itself has preserved soft tissue extending well beyond the margins of the skeleton. This, combined with the already flattened appearance of the tail vertebrae, suggests that a ridge of skin may have extended from the top and bottom of the tail creating a small fin. Webbed feet are also preserved.[8] Skin impressions of Champsosaurus have also been reported, they consist of small (0.6-0.1 mm) pustulate and rhomboid scales, with the largest scales being located on the lateral sides of the body, decreasing in size dorsally, no osteoderms were present.[11] The Menat specimen of Lazarussuchus preserves some remnants of soft tissue, but no scales, which shows that the hindfoot (pes) was not webbed, and a dark stained region with a crenellated edge is present above the caudal vertebrae of the tail, suggestive of a crest similar to those found in some living reptiles, like the tuatara, lizards and crocodiles.[12]
Tracks
Tracks from the Early Cretaceous (Albian) of South Korea, given the ichnotaxon name Novapes ulsanensis have been attributed to choristoderans, based on the similarity of the pentadactyl (five fingered) preserved tracks to the foot morphology of Monjurosuchus. The tracks preserve traces of webbing between the digits. The authors of the study proposed based on the spacing of the prints, that choristoderans could "high walk" like modern crocodilians.[13] Tracks attributed to neochoristoderans dubbed Champsosaurichnus parfeti have also been reported from the Late Cretaceous Laramie Formation of the United States, though only two prints are present and it is not possible to distinguish between a manus (forefoot) or pes (hindfoot).[14]
Paleobiology
Choristoderes are exclusively found in freshwater deposits, often associated with turtles, fish, frogs, salamanders and crocodyliformes. They appear to have been almost exclusively found in warm temperate climates, with the range of neochoristoderes extending to the high Canadian Arctic during Coniacian-Santonian stages of the Late Cretaceous (~89-83 Million years ago), a time of extreme warmth. Due to the morphological simililarities between choristoderes and crocodyliformes, it has often been assumed that they existed in competition. However "non-neochoristoderes" are morphologically dissimilar to aquatic crocodyliformes and were more likely in competition with other taxa. For the more crocodile-like neochoristoderes, there appears to have been niche differentiation, with gharial-like neochoristoderans occurring in association with blunt snouted crocodyliformes, but not in association with long snouted forms.[4]
Diet
Direct dietary evidence for most choristoderes is lacking. Neochoristoderans are presumed to have been piscivorous.[11] Preserved gut contents of a Monjurosuchus specimen appear to show arthropod cuticle fragments,[10] Another specimen of Monjurosuchus has been found with preserved skulls of seven juvenile individuals within the abdominal cavity. This has been proposed to represent evidence of cannibalism.[15] However, this proposal has been criticised by other authors, who suggest it is more likely that they represent late-stage embryos.[16]
Reproduction
A specimen of Hyphalosaurus has been found with 18 fully developed embryos within the mothers body, suggesting that they were viviparous.[17] but it has also been shown that they possessed soft shelled eggs, similar to those of lepidosaurs.[18] A possible explanation for this is that Hyphalosaurus was ovoviparous, with the thin shelled eggs hatching immediately after they were laid, presumably on land.[19] In Champsosaurus, it has been suggested that adult females could crawl ashore to lay eggs on land, with males and juveniles appearing to be incapable of doing so, based on the presumably sexually dimorphic fusion of the sacral vertebrae and possession of more robust limb bones in presumed females.[20] A skeleton of Philydrosaurus has been found with associated post-hatchling stage juveniles, suggesting that they engaged in post-hatching parental care.[19]
Classification and phylogeny
Choristoderes are universally agreed to be members of Neodiapsida, but their exact placement in the clade is uncertain, due to their mix of primitive and derived features, and a long ghost lineage (absence of a fossil record) after their split from other reptiles. In a 2016 analysis of neodiapsid relationships they were recovered as members of Sauria, in a polytomy with Lepidosauromorpha and Archosauromorpha with being the earliest diverging members of either group also being plausible.[21] Historically, the phylogenetics of Choristodera were unclear, with the neochoristoderes being recovered as a well-supported clade, but the relationships of the "non-neochoristoderes" being poorly resolved.[6] However, during the 2010's, the "non-neochoristoderes" from the Early Cretaceous of Asia (with the exception of Heishanosaurus) alongside Lazarussuchus from the Cenozoic of Europe were recovered (with weak support) as belonging to a monophyletic clade, which were informally named the "Allochoristoderes" by Dong and colleagues in 2020, characterised by the shared trait of completely closed lower temporal fenestrae, with Cteniogenys from the Middle-Late Jurassic of Europe and North America being consistently recovered as the basalmost choristodere.[22] The long necked "non-neochoristoderes" Shokawa and Hyphalosaurus have often been recovered as a clade, dubbed the Hyphalosauridae by Gao and Fox in 2005.[23] The finding of more complete material of the previously fragmentary Khurendukhosaurus shows that it also has a long neck, and it has also been recovered as part of the clade.[24]
Phylogeny from the analysis of Dong and colleagues (2020):[22]
| Choristodera |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Evolutionary history

Choristoderes must have diverged from all other known reptile groups prior to the end of the Permian period, over 250 million years ago, based on their primitive phylogenetic position. In 2015, Rainer R. Schoch reported a new small (~ 20 cm long) diapsid from the Middle Triassic (Ladinian) Lower Keuper of Southern Germany, known from both cranial and postcranial material, which he claimed represented the oldest known choristodere.[25] Pachystropheus from the Late Triassic (Rhaetian) of Britain has been suggested to be a choristodere, but cannot be referred in confidence to the group as it lacks cranial material, on which most diagnostic characters of Choristodera are based. The oldest unequivocal choristoderan is the small lizard-like Cteniogenys, the oldest known remains of which are known from the late Middle Jurassic (Bathonian ~168-166 million years ago) of Britain, with remains also known from the Upper Jurassic of Portugal and the United States, with broadly similar remains also known from the late Middle Jurassic (Callovian) of Kyrgyzstan[4] and the Bathonian of western Siberia,[26] European Russia,[27] as well as possibly the Bathonian of North Africa.[1]
Choristoderes underwent a major evolutionary radiation in the Lower Cretaceous of Asia, which represents the high point of choristoderan diversity, including the first records of the gharial-like Neochoristodera, which appear to have evolved in the regional absence of aquatic neosuchians. The only remains outside of Asia known from this time period is a partial femur from the Yellow Cat Member of the Cedar Mountain Formation in North America, they appear to be absent from the well sampled European localities of the Berriasian aged Purbeck Group, England and the Barremian aged La Huérguina Formation, Spain. In the Late Cretaceous, the neochoristodere Champsosaurus is abundant across North America.[4] Vertebrae from the Cenomanian of Germany[28] and the Campanian aged Grünbach Formation of Austria indicate the presence of choristoderes in Europe during this time period, however, there are no records from Asia. Fragmentary remains found in the Campanian aged Oldman and Dinosaur Park formations in Alberta, Canada, also possibly suggest the presence of small bodied "non-choristoderes" in North America the Late Cretaceous. Champsosaurus survived the K-Pg extinction, alongside the addition of fellow neochoristodere Simoedosaurus were found in Europe, Asia and North America during the Paleocene, however they became extinct during the early Eocene.[4] Small bodied "non-choristoderes", which are absent from the fossil record after the Early Cretaceous (with the exception of the possible North American remains), unexpectedly reappear in the form of the lizard-like Lazarussuchus from the late Paleocene of France.[12] The European endemic Lazarussuchus is the last known choristodere, survivng the extinction of neochoristoderes during the Eocene, with the last known species being L. dvoraki from the Early Miocene of the Czech Republic.[4][29] Remains of Lazarussuchus have also been reported the late Miocene (~11.6 million years ago) of southern Germany.[30]
References
- Haddoumi H, Allain R, Meslouh S, Metais G, Monbaron M, Pons D, et al. (2016). "Guelb el Ahmar (Bathonian, Anoual Syncline, eastern Morocco): First continental flora and fauna including mammals from the Middle Jurassic of Africa" (PDF). Gondwana Research. 29 (1): 290–319. Bibcode:2016GondR..29..290H. doi:10.1016/j.gr.2014.12.004.
- Cope ED (1876). "On some extinct reptiles and Batrachia from the Judith River and Fox Hills beds of Montana". Proceedings of the Academy of Natural Sciences of Philadelphia. 1876: 340–359.
- Cope ED (1884). "The Choristodera". American Naturalist. 18: 815–817.
- Matsumoto R, Evans SE (2010). "Choristoderes and the freshwater assemblages of Laurasia". Journal of Iberian Geology. 36 (2): 253–274. doi:10.5209/rev_jige.2010.v36.n2.11.
- Evans SE, Hecht MK (1993). "A History of an Extinct Reptilian Clade, the Choristodera: Longevity, Lazarus-Taxa, and the Fossil Record". In Hecht M, MacIntyre RJ, Clegg MT (eds.). Evolutionary Biology. Evolutionary Biology. Boston, MA: Springer US. pp. 323–338. doi:10.1007/978-1-4615-2878-4_8. ISBN 978-1-4615-2878-4.CS1 maint: date and year (link)
- Matsumoto R, Dong L, Wang Y, Evans SE (2019). "The first record of a nearly complete choristodere (Reptilia: Diapsida) from the Upper Jurassic of Hebei province, People's Republic of China" (PDF). Journal of Systematic Palaeontology. 17 (12): 1031–1048. doi:10.1080/14772019.2018.1494220. S2CID 92421503.
- Dudgeon TW, Maddin HC, Evans DC, Mallon JC (April 2020). "Computed tomography analysis of the cranium of Champsosaurus lindoei and implications for the choristoderan neomorphic ossification". Journal of Anatomy. 236 (4): 630–659. doi:10.1111/joa.13134. PMC 7083570. PMID 31905243.
- Gao KQ, Ksepka DT (June 2008). "Osteology and taxonomic revision of Hyphalosaurus (Diapsida: Choristodera) from the Lower Cretaceous of Liaoning, China". Journal of Anatomy. 212 (6): 747–68. doi:10.1111/j.1469-7580.2008.00907.x. PMC 2423398. PMID 18510504.
- Matsumoto R, Evans SE (March 2016). "Morphology and function of the palatal dentition in Choristodera". Journal of Anatomy. 228 (3): 414–29. doi:10.1111/joa.12414. PMC 5341546. PMID 26573112.
- Keqin G, Evans S, Qiang J, Norell M, Shu'An J (25 September 2000). "Exceptional fossil material of a semi-aquatic reptile from China: the resolution of an enigma". Journal of Vertebrate Paleontology. 20 (3): 417–421. doi:10.1671/0272-4634(2000)020[0417:EFMOAS]2.0.CO;2.
- Erickson BR (June 1985). "Aspects of some anatomical structures of Champsosaurus (Reptilia: Eosuchia)". Journal of Vertebrate Paleontology. 5 (2): 111–127. doi:10.1080/02724634.1985.10011849.
- Matsumoto R, Buffetaut E, Escuillie F, Hervet S, Evans SE (2013). "New material of the choristodere Lazarussuchus (Diapsida, Choristodera) from the Paleocene of France". Journal of Vertebrate Paleontology. 33 (2): 319. doi:10.1080/02724634.2012.716274.
- Lee YN, Kong DY, Jung SH (September 2020). "The first possible choristoderan trackway from the Lower Cretaceous Daegu Formation of South Korea and its implications on choristoderan locomotion". Scientific Reports. 10 (1): 14442. doi:10.1038/s41598-020-71384-1. PMC 7468130. PMID 32879388.
- Lockley, Martin G.; Hunt, Adrian P. (14 September 1995). "Ceratopsid tracks and associated ichnofauna from the Laramie Formation (Upper Cretaceous: Maastrichtian) of Colorado". Journal of Vertebrate Paleontology. 15 (3): 592–614. doi:10.1080/02724634.1995.10011251. ISSN 0272-4634.
- Wang, Xiaolin; Miao, Desui; Zhang, Yuguang (1 February 2005). "Cannibalism in a semi-aquatic reptile from the Early Cretaceous of China". Chinese Science Bulletin. 50 (3): 282–284. doi:10.1007/BF02897540. ISSN 1861-9541.
- Gao, Ke-Qin; Ksepka, Daniel; Lianhai, Hou; Ye, Duan; Dongyu, Hu (January 2007). "Cranial morphology of an Early Cretaceous Monjurosuchid (Reptilia: Diapsida) from Liaoning Province of China and evolution of the choristoderan palate". Historical Biology. 19 (3): 215–224. doi:10.1080/08912960601106391. ISSN 0891-2963.
- Ji Q, Wu XC, Cheng YN (April 2010). "Cretaceous choristoderan reptiles gave birth to live young". Die Naturwissenschaften. 97 (4): 423–8. doi:10.1007/s00114-010-0654-2. PMID 20179895.
- Hou LH, Li PP, Ksepka DT, Gao KQ, Norell MA (April 2010). "Implications of flexible-shelled eggs in a Cretaceous choristoderan reptile". Proceedings. Biological Sciences. 277 (1685): 1235–9. doi:10.1098/rspb.2009.2035. PMC 2842823. PMID 20018793.
- Lü J, Kobayashi Y, Deeming DC, Liu Y (20 October 2014). "Post-natal parental care in a Cretaceous diapsid from northeastern China". Geosciences Journal. 19 (2): 273–280. doi:10.1007/s12303-014-0047-1.
- Katsura Y (2007). "Fusion of sacrals and anatomy in Champsosaurus(Diapsida, Choristodera)". Historical Biology. 19 (3): 263–271. doi:10.1080/08912960701374659. S2CID 84966652.
- Ezcurra MD (28 April 2016). "The phylogenetic relationships of basal archosauromorphs, with an emphasis on the systematics of proterosuchian archosauriforms". PeerJ. 4: e1778. doi:10.7717/peerj.1778. ISSN 2167-8359.
- Dong, Liping; Matsumoto, Ryoko; Kusuhashi, Nao; Wang, Yuanqing; Wang, Yuan; Evans, Susan E. (2 August 2020). "A new choristodere (Reptilia: Choristodera) from an Aptian–Albian coal deposit in China". Journal of Systematic Palaeontology. 18 (15): 1223–1242. doi:10.1080/14772019.2020.1749147. ISSN 1477-2019.
- Gao, Ke-Qin; Fox, Richard C. (16 November 2005). "A new choristodere (Reptilia: Diapsida) from the Lower Cretaceous of western Liaoning Province, China, and phylogenetic relationships of Monjurosuchidae". Zoological Journal of the Linnean Society. 145 (3): 427–444. doi:10.1111/j.1096-3642.2005.00191.x. ISSN 1096-3642.
- Matsumoto, Ryoko; Tsogtbaatar, Khishigjav; Ishigaki, Shinobu; Tsogtbaatar, Chinzorig; Enkhtaivan, Zorig; Evans, Susan (2019). "Revealing body proportions of the enigmatic choristodere Khurendukhosaurus from Mongolia". Acta Palaeontologica Polonica. 64. doi:10.4202/app.00561.2018.
- Schoch, R.R (2015). "Reptilien" (PDF). Der Lettenkeuper: ein Fenster in die Zeit vor den Dinosauriern (in German). Staatliches Museum für Naturkunde Stuttgart. pp. 231–264.
- "Middle Jurassic vertebrate assemblage of Berezovsk coal mine in western Siberia (Russia)". Global Geology. 19 (4): 187–204. 2016. doi:10.3969/j.issn.1673-9736.2016.04.01.
- Pashchenko, D. I.; Kuzmin, I. T.; Sennikov, A. G.; Skutschas, P. P.; Efimov, M. B. (1 September 2018). "On the Finding of Neosuchians (Neosuchia, Crocodyliformes) in the Middle Jurassic (Bathonian) Deposits of the Moscow Region". Paleontological Journal. 52 (5): 550–562. doi:10.1134/S0031030118050118. ISSN 1555-6174.
- Reiss S, Scheer U, Sachs S, Kear BP (13 December 2018). "Filling the biostratigraphical gap: First choristoderan from the Lower - mid-Cretaceous interval of Europe". Cretaceous Research. 96: 135–141. doi:10.1016/j.cretres.2018.12.009. ISSN 0195-6671.
- Evans SE, Klembara J (2005). "A choristoderan reptile (Reptilia: Diapsida) from the Lower Miocene of northwest Bohemia (Czech Republic)". Journal of Vertebrate Paleontology. 25 (1): 171–184. doi:10.1671/0272-4634(2005)025[0171:ACRRDF]2.0.CO;2. ISSN 0272-4634.
- Böhme M, Spassov N, Fuss J, Tröscher A, Deane AS, Prieto J, et al. (November 2019). "Supplementary Information: A new Miocene ape and locomotion in the ancestor of great apes and humans" (PDF). Nature. 575 (7783): 489–493. doi:10.1038/s41586-019-1731-0. PMID 31695194.
Further reading
- de Braga M, Rieppel O (1997). "Reptile phylogeny and the interrelationships of turtles". Zoology – J. Linnean Soc. 120 (3): 281–354. doi:10.1111/j.1096-3642.1997.tb01280.x.
- Erickson BR (1972). The Lepidosaurian Reptile Champsosaurus in North America. Paleontology, Monograph. 1. Science Museum of Minnesota.
- Evans SE, Hecht MK (1993). "A history of an extinct reptilian clade, the Choristodera: Longevity, Lazarus taxa, and the fossil record". Evolutionary Biology. 27: 323–338.
- Gao K, Fox RC (1998). "New choristoderes (Reptilia: Diapsida) from the Upper Cretaceous and Palaeocene, Alberta and Saskatchewan, Canada, and phylogenetic relationships of Choristodera". Zoology – J. Linnean Soc. 124 (4): 303–353. doi:10.1111/j.1096-3642.1998.tb00580.x.
- Matsumoto R, Suzuki S, Tsogtbaatar K, Evans SE (February 2009). "New material of the enigmatic reptile Khurendukhosaurus (Diapsida: Choristodera) from Mongolia". Die Naturwissenschaften. 96 (2): 233–42. Bibcode:2009NW.....96..233M. doi:10.1007/s00114-008-0469-6. PMID 19034405. S2CID 13542692.
- Ksepka D, Gao K, Norell MA (2005). "A new choristodere from the Cretaceous of Mongolia". American Museum Novitates. 3468: 1–22. doi:10.1206/0003-0082(2005)468<0001:ancftc>2.0.co;2. hdl:2246/2778.
- Storrs GW, Gower DJ (1993). "The earliest possible choristodere (Diapsida) and gaps in the fossil record of semi-aquatic reptiles". Journal of the Geological Society [of America]. 150 (6): 1103–1107. Bibcode:1993JGSoc.150.1103S. doi:10.1144/gsjgs.150.6.1103. S2CID 86088809.
External links
- "Choristodera page". Palæos. Archived from the original on 16 April 2007.
- "Choristodera-champsosaurs". Mikko's Phylogeny Archive.
- "Archosauria: Systematics". U.C. Berkeley.
- "Two-Headed Reptile Fossil Found". BBC News.
- "Ghost lineages". Archived from the original on 18 April 2008. — brief article on the fossil record of choristoderes