Dabrafenib
Dabrafenib, sold under the brand name Tafinlar, is a medication for the treatment of cancers associated with a mutated version of the gene BRAF. Dabrafenib acts as an inhibitor of the associated enzyme B-Raf, which plays a role in the regulation of cell growth. Dabrafenib has clinical activity with a manageable safety profile in clinical trials of phase 1 and 2 in patients with BRAF (V600)-mutated metastatic melanoma.[1][2]
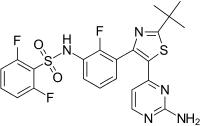 | |
| Clinical data | |
|---|---|
| Trade names | Tafinlar |
| Other names | GSK-2118436 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613038 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.965 |
| Chemical and physical data | |
| Formula | C23H20F3N5O2S2 |
| Molar mass | 519.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Approvals and indications
The US Food and Drug Administration initially approved dabrafenib as a single agent treatment for patients with BRAF V600E mutation-positive advanced melanoma on May 29, 2013.[3][4] Dabrafenib was approved for use in the European Union in August 2013.[5]
Clinical trial data demonstrated that resistance to dabrafenib and other BRAF inhibitors occurs within six to seven months.[6] To overcome this resistance, the BRAF inhibitor dabrafenib was combined with the MEK inhibitor trametinib.[6] On January 8, 2014, the FDA approved this combination of dabrafenib and trametinib for BRAF V600E/K-mutant metastatic melanoma.[7][8] On May 1, 2018, the FDA approved the combination dabrafenib/trametinib as an adjuvant treatment for BRAF V600E-mutated, stage III melanoma after surgical resection based on the results of the COMBI-AD phase 3 study,[9] making it the first oral chemotherapy regimen that prevents cancer relapse for node positive, BRAF-mutated melanoma.[10]
In April 2017, the European Union approved the combination of dabrafenib with trametinib for BRAF V600-positive advanced or metastatic non small-cell lung cancer (NSCLC).[11][12][5]
References
- Gibney, G. T.; Zager, J. S. (2013). "Clinical development of dabrafenib in BRAF mutant melanoma and other malignancies". Expert Opinion on Drug Metabolism & Toxicology. 9 (7): 893–9. doi:10.1517/17425255.2013.794220. PMID 23621583. S2CID 207491581.
- Huang, T.; Karsy, M.; Zhuge, J.; Zhong, M.; Liu, D. (2013). "B-Raf and the inhibitors: From bench to bedside". Journal of Hematology & Oncology. 6: 30. doi:10.1186/1756-8722-6-30. PMC 3646677. PMID 23617957.
- "Drug Approval Package: Tafinlar (dabrafenib) Capsules NDA #202806". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 10 April 2020.
- "GSK melanoma drugs add to tally of U.S. drug approvals". Reuters. May 30, 2013.
- "Tafinlar EPAR". European Medicines Agency (EMA). Retrieved 10 April 2020.
- Flaherty, Keith T.; Infante, Jeffery R.; Daud, Adil; Gonzalez, Rene; Kefford, Richard F.; Sosman, Jeffrey; Hamid, Omid; Schuchter, Lynn; Cebon, Jonathan; Ibrahim, Nageatte; Kudchadkar, Ragini; Burris, Howard A.; Falchook, Gerald; Algazi, Alain; Lewis, Karl; Long, Georgina V.; Puzanov, Igor; Lebowitz, Peter; Singh, Ajay; Little, Shonda; Sun, Peng; Allred, Alicia; Ouellet, Daniele; Kim, Kevin B.; Patel, Kiran; Weber, Jeffrey (November 1, 2012). "Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations". New England Journal of Medicine. 367 (18): 1694–703. doi:10.1056/NEJMoa1210093. PMC 3549295. PMID 23020132.
- "Dabrafenib/Trametinib Combination Approved for Advanced Melanoma". OncLive. January 9, 2013.
- Maverakis E; Cornelius LA; Bowen GM; Phan T; Patel FB; Fitzmaurice S; He Y; Burrall B; Duong C; Kloxin AM; Sultani H; Wilken R; Martinez SR; Patel F (2015). "Metastatic melanoma – a review of current and future treatment options". Acta Derm Venereol. 95 (5): 516–524. doi:10.2340/00015555-2035. PMID 25520039.
- Long, Georgina V.; Hauschild, Axel; Santinami, Mario; Atkinson, Victoria; Mandalà, Mario; Chiarion-Sileni, Vanna; Larkin, James; Nyakas, Marta; Dutriaux, Caroline; Haydon, Andrew; Robert, Caroline; Mortier, Laurent; Schachter, Jacob; Schadendorf, Dirk; Lesimple, Thierry; Plummer, Ruth; Ji, Ran; Zhang, Pingkuan; Mookerjee, Bijoyesh; Legos, Jeff; Kefford, Richard; Dummer, Reinhard; Kirkwood, John M. (9 November 2017). "Adjuvant Dabrafenib plus Trametinib in Stage III-Mutated Melanoma". New England Journal of Medicine. 377 (19): 1813–1823. doi:10.1056/NEJMoa1708539. PMID 28891408. S2CID 205102412.
- "FDA Approves Adjuvant Combo for BRAF+ Melanoma". www.medscape.com. WebMD LLC. Retrieved 2 May 2018.
- "EU Approves Dabrafenib/Trametinib Combination in BRAF+ NSCLC". Targeted Oncology. 4 April 2017. Retrieved 10 April 2020.
- "Mekinist EPAR". European Medicines Agency (EMA). Retrieved 10 April 2020.
Further reading
- Dean L (2017). "Dabrafenib Therapy and BRAF and G6PD Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28809523. Bookshelf ID: NBK447415.
