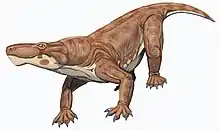
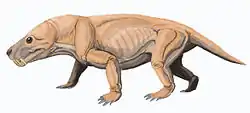
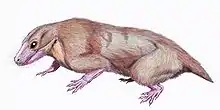
Euchambersia
Euchambersia is a genus of therocephalian therapsid that lived during the Late Permian, approximately 255 million years ago, in what is now South Africa. The genus contains a single species, Euchambersia mirabilis, named by paleontologist Robert Broom in 1931 from a skull missing the lower jaws; a second skull, belonging to an immature individual, was later described. It is a member of the family Akidnognathidae, which historically has also been referred by as the synonymous Euchambersiidae (named after Euchambersia).
| Euchambersia | |
|---|---|
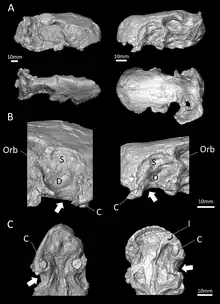 | |
| CT scans of the skulls belonging to the type (right) and second (left) specimens | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Therapsida |
| Suborder: | †Therocephalia |
| Family: | †Akidnognathidae |
| Genus: | †Euchambersia Broom, 1931 |
| Type species | |
| †Euchambersia mirabilis Broom, 1931 | |
Euchambersia was a small and short-snouted therocephalian, possessing large canines as is typical of the group. However, it is notable among therocephalians for possessing ridges on its canines and a large indentation in the side of the skull. Under the erroneous assumption that the canines are grooved instead of ridged, it has been proposed that these structures supported a venom delivery mechanism. More recently, the internal structure of the skull of Euchambersia has been used as stronger evidence in favour of the hypothesis that it was venomous; other possibilities, such as the indentation supporting some sort of sensory organ, still remain plausible.
Discovery and naming
The type specimen of Euchambersia was found by Robert Broom on the South African farm of Vanwyksfontein, owned by a Mr. Greathead, near the town of Norvalspont.[1] It consists of a single, distorted skull, catalogued as NHMUK R5696, which was described by Broom in 1931. A second, smaller skull, with the specimen number BP/1/4009, was found in 1966[2] and described by James Kitching in 1977.[3] Both specimens are missing the lower jaw. They originated from the same general layer of rock, in the upper Cistecephalus Assemblage Zone of the Beaufort Group within the Karoo Supergroup.[3] The Cistecephalus AZ has been dated to the Wuchiapingian stage of the Late Permian,[4] between 256.2 and 255.2 Mya.[5]
Broom named the genus Euchambersia, which he considered "the most remarkable therocephalian ever discovered", after the eminent Scottish publisher and evolutionary thinker Robert Chambers, whose Vestiges of the Natural History of Creation was considered by Broom to be "a very remarkable work" though "sneered at by many".[1]
Description

Euchambersia was small and short-snouted for a therocephalian, with the type skull having a reconstructed length of approximately 116 millimetres (4.6 in), accounting for crushing and deformation in the fossil. The second known skull belonged to a smaller individual, with a length of 80 millimetres (3.1 in); it was probably immature, judging by the lack of fusion in the skull.[2]
According to the initial description, the eye socket of Euchambersia was rather small. The branches of the postorbital and jugal that usually surround the back and bottom of the eye socket in therocephalians appear to be either very reduced or absent entirely. Meanwhile, the top of the eye socket is formed by the prefrontal, and the frontal is also small. The skull does not bear a pineal foramen. Like Whaitsia, the pterygoid and palatine of the palate are not separated from the transpalatine, further to the side of the jaw, by any sort of opening.[1]
Teeth
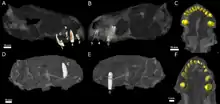
Although the skulls are incompletely preserved, CT scanning suggests that each premaxilla held five incisors, with the sockets becoming progressively larger from the first to the fifth incisor. Like other theriodonts, the crowns of the incisors are conical; they also lack serrations, unlike gorgonopsians and scylacosaurian therocephalians. The interior edge of the incisors seems to be slightly concave, and the back edge appears to have a ridge. The smaller specimen has a displaced incisor preserved within its nasal cavity; it is more strongly recurved and has wear marks on its top edge, suggesting that it is probably a lower incisor. Its fourth incisor also has a replacement tooth growing behind it, accompanied by resorption of the root.[2]
The type specimen preserves the right canine.[2] Like other therocephalians, the canine of Euchambersia was very large, resulting in a specialized predatory lifestyle that incorporates a sabertooth bite into prey killing.[6] It is round in cross-section,[3] and bears a prominent ridge on the side of its front surface. Immediately beside this ridge is a shallow depression that becomes wider near the top of the tooth, which is probably the same structure as the groove interpreted by some authors.[2][7] Theriodonts usually replace their teeth in an alternating[8] (or distichial) pattern,[9][10] such that the canine tooth is always functional; both skulls of Euchambersia show no sign of any replacement teeth developing, suggesting that Euchambersia was reliant on having both canines present and functional simultaneously.[2]
Maxillary fossa and associated canals
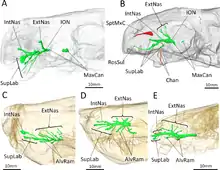
Behind the incisors and canines, there were no additional teeth in the jaw. Where teeth would be located in therocephalians that do have teeth behind the canines, there is instead a large depression, or fossa, on the side of the maxilla, which is also bounded below by part of the lacrimal and possibly part of the jugal.[1] This fossa is 48% the length of the jaw in the type specimen, and 38% in the second skull. In both skulls, this fossa is divided into two parts: a shallower ridge on top, and a larger and deeper depression on the bottom. A wide furrow beginning behind the canine contacts the bottom of the fossa and then passes into the interior of the mouth. The bottom portion of the fossa is strongly pitted and bears a small opening, or foramen, on both the front and back surfaces.[2]
CT scanning shows that these openings lead to canals that connect to the trigeminal nerve, which controls facial sensitivity. The forward-directed canal also splits into the three main branches of the infraorbital nerve,[11] all of which connect to the socket of the canine; the junction occurs about 3–6 millimetres (0.12–0.24 in) along the canal, another point of variation between the two skulls. The top branch, the external nasal ramus, splits into four branches in the type skull, but it does not split in the second skull. In other therapsids like Thrinaxodon, Bauria, and Olivierosuchus, the external nasal ramus generally splits into three or more branches. All of these canals would have brought nerves and nutrient-rich tissue to the root of the canines and the rest of the upper jaw.[2][11]
Classification
In 1934, Euchambersia was assigned to the newly named family Euchambersiidae by Lieuwe Dirk Boonstra.[12][13] Boonstra initially misspelt the name as Euchambersidae (which is improper Latin), and was subsequently corrected by Friedrich von Huene in 1940. Euchambersiidae was initially considered to be separate from the families Moschorhinidae and Annatherapsididae; in 1974, Christiane Mendez recognized these groups as closely related subfamilies (renamed Annatherapsidinae, Moschorhininae and Euchambersiinae) within the wider group of her redefined Moschorhinidae (although she also referred to it as Annatherapsididae).[14]
The 1986 phylogenetic analysis of James Hopson and Herb Barghusen supported Mendez's hypothesis of three subfamilies within Moschorhinidae, but they elected to use the name Euchambersiidae. In 2009, Adam Huttenlocker and colleagues argued that the names Annatherapsididae, Moschorhinidae, and Euchambersiidae are junior synonyms of Akidnognathidae, since Akidnognathus (which also belongs in the same family) was named first before any other member of the family.[14] This name has reached wider acceptance among researchers.[14][15][16] Huttenlocker et al. also later redefined Moschorhininae as all of Akidnognathidae save for Annatherapsidus and Akidnognathus.[17]
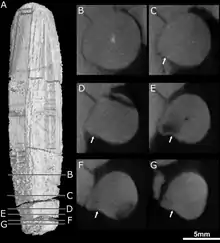
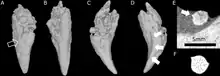
In 2008, Ivakhnenko included the Akidnognathidae (as the Euchambersiidae) as the sister group of the family Whaitsiidae in the superfamily Whaitsioidea.[13] However, other researchers do not include the Akidnognathidae in the Whaitsioidea. Phylogenies by Huttenlocker et al. found that the Akidnognathidae was instead closest to the Chthonosauridae, with the two forming the sister group to the group containing the Whaitsioidea and the Baurioidea. The topology recovered by the 2016 analysis of Huttenlocker et al. is shown below.[17]
| Therocephalia |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Paleobiology

Venom
The large maxillary fossae of Euchambersia have been continual subjects of debate regarding their function. However, most researchers agree that they held some sort of secretory gland. While Broom initially argued that the fossae may have contained the parotid salivary glands,[1] this proposal was rejected by Boonstra and J.P. Lehman, who noted that the parotid glands tend to be placed behind the eye; they respectively suggested that the fossae held modified lacrimal glands and Harderian glands.[2] However, the latter is also unlikely because Harderian glands are usually placed inside the eye socket. Franz Nopcsa suggested that the maxillary fossae housed venom glands (which may have been derived from lacrimal glands), with the ridged canines and the notches behind the canines allowing the venom to flow passively into the victim's bloodstream.[18] This hypothesis was widely accepted throughout the 20th century[15][19][20][21] and the characteristic morphology of Euchambersia was used to support possible venom-bearing adaptations among various other prehistoric animals,[7][13][22][23] including the therocephalian Ichibengops.[24]
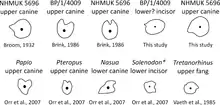
Much of this acceptance has been based on the erroneous assumption that the canines are grooved instead of ridged;[3] grooved canines in Euchambersia would parallel the fangs of various venomous snakes as well as the venom-delivering incisors of the living solenodons.[21] This interpretation, which has consistently appeared in literature published after 1986, was determined by Julien Benoit to be the result of the propagation of Broom's overly reconstructed diagram of the skull, without the context of the actual specimens. He thus considered it necessary to re-evaluate the hypothesis of a venomous bite in Euchambersia.[3] Additionally, Benoit argued that grooved and ridged canines are not necessarily associated with venomous animals either, as shown by their presence in hippopotami, muntjacs, and baboons, in which they play a role in grooming or sharpening the teeth;[3][21][25] in the latter two, ridged canines are also accompanied by a distinct fossa in front of the eye, which is entirely unconnected with venom.[21][26] Furthermore, grooved and ridged teeth in non-venomous snakes are used to reduce suctional drag when capturing slippery prey like fish or invertebrates.[27]
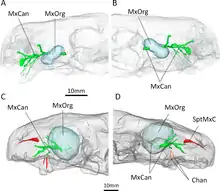
CT scanning of the known specimens of Euchambersia by Benoit and colleagues was subsequently used to provide more concrete support in favour of the venom hypothesis. The canals leading into and from the maxillary fossae, as revealed by the scans, would primarily have supported the trigeminal nerve as well as blood vessels.[28][29][30] However, the fact that the canals also directly lead to the root of the canines would suggest that they had a secondary role in venom delivery. In all, Euchambersia seems to have had a venom gland (housed in the maxillary fossae), a delivery mechanism of the venom (the maxillary canals), and an instrument by which a wound for venom delivery can be inflicted (the ridged canines), which satisfy the criteria of a venomous animal as defined by Wolfgang Bücherl.[31] Benoit et al. noted that this does not conclusively demonstrate that Euchambersia was actually venomous, especially given the previously stated objections. Additionally, there are no living animals with a delivery system analogous to the proposed system for Euchambersia (most deliver venom through the lower jaw,[32][33] while snakes have specialized ducts.[2][34]
An alternate hypothesis suggested by Benoit et al. involves some kind of sensory organ occupying the maxillary fossa. Uniquely among therapsids,[11] the canal within the maxilla is exposed on the back side of the maxillary fossa, which implies that the canal, carrying the trigeminal nerve, would probably have extended across the fossa, outside of the outline of the skull. Benoit et al. hypothesized that the fossa may have supported a specialized sensory organ analogous to the pit organ of pit vipers and some other snakes,[35] or alternatively a ganglion of nerve cells. It is also possible that this organ functioned as a replacement for the parietal eye in Euchambersia, like the pit organ does in pit vipers.[36] However, such an expanded sensory organ would be unprecedented among tetrapods, and the few other therocephalians that also lack a parietal eye do not have a maxillary fossa either.[37] Thus, Benoit et al. considered the venom hypothesis as being more plausible.[2]
Paleoecology
The Cistecephalus Assemblage Zone, from where Euchambersia is known, represents a floodplain that was covered in many small, relatively straight streams. The water level in these streams was probably seasonally dependent.[4] Judging from pollen preserved in the Cistecephalus AZ, the pollen taxon Pityosporites (which probably originated from a plant similar to Glossopteris) was very common, forming some 80% to 90% of the pollen discovered (although the prevalent sediments would not have been ideal for pollen preservation).[38]
In the Cistecephalus AZ, other co-occurring therocephalians included Hofmeyria, Homodontosaurus, Ictidostoma, Ictidosuchoides, Ictidosuchops, Macroscelesaurus, Polycynodon, and Proalopecopsis. More numerous, however, were the gorgonopsians, which included Aelurognathus, Aelurosaurus, Aloposaurus, Arctognathus, Arctops, Cerdorhinus, Clelandina, Cyonosaurus, Dinogorgon, Gorgonops, Lycaenops, Leontocephalus, Pardocephalus, Prorubidgea, Rubidgea, Scylacops, Scymnognathus, and Sycosaurus.[4]
By far the most abundant herbivore was the dicynodont Diictodon, with over 1900 known specimens from the Cistecephalus AZ. Other dicynodonts included Aulacephalodon, Cistecephalus, Dicynodon, Dicynodontoides, Digalodon, Dinanomodon, Emydops, Endothiodon, Kingoria, Kitchinganomodon, Oudenodon, Palemydops, Pelanomodon, Pristerodon, and Rhachiocephalus. The biarmosuchians Lemurosaurus, Lycaenodon, Paraburnetia, and Rubidgina were also present, along with the cynodonts Cynosaurus and Procynosuchus. Non-synapsids included the archosauromorph Younginia; the parareptilians Anthodon, Milleretta, Nanoparia, Owenetta, and Pareiasaurus; and the temnospondyl Rhinesuchus.[4]
See also
References
- Broom, R. (1931). "Notices of some new genera and species of Karroo fossil reptiles". Records of the Albany Museum. 4 (1): 161–166.
- Benoit, J.; Norton, L.A.; Manger, P.R.; Rubidge, B.S. (2017). "Reappraisal of the envenoming capacity of Euchambersia mirabilis (Therapsida, Therocephalia) using μCT-scanning techniques". PLoS ONE. 12 (2): e0172047. Bibcode:2017PLoSO..1272047B. doi:10.1371/journal.pone.0172047. PMC 5302418. PMID 28187210.
- Benoit, J. (2016). "A review of the "venomous therocephalian" hypothesis and how multiple re-portrayals of Euchambersia have influenced its success and vice versa". Bulletin de la Société Géologique de France. 187 (4): 217–224. doi:10.2113/gssgfbull.187.4-5.217.
- Smith, R.; Rubidge, B.; van der Walt, Merrill (2012). "Therapsid Biodiversity Patterns and Palaeoenvironments of the Karoo Basin, South Africa". In Chinsamy-Turan, A. (ed.). Forerunners of Mammals: Radiation, Histology, Biology. Bloomington: Indiana University Press. pp. 31–64. ISBN 978-0-253-00533-5.
- Rubidge, B.S.; Erwin, D.H.; Ramezani, J.; Bowring, S.A.; de Klerk, W.J. (2013). "High-precision temporal calibration of Late Permian vertebrate biostratigraphy: U-Pb zircon constraints from the Karoo Supergroup, South Africa". Geology. 41 (3): 363–366. Bibcode:2013Geo....41..363R. doi:10.1130/G33622.1.
- Andersson, K.; Norman, D.; Werdelin, L. (2011). "Sabretoothed Carnivores and the Killing of Large Prey". PLoS ONE. 6 (10): e24971. Bibcode:2011PLoSO...624971A. doi:10.1371/journal.pone.0024971. PMC 3198467. PMID 22039403.
- Sues, H.-D. (1991). "Venom-conducting teeth in a Triassic reptile". Nature. 351 (6322): 141–143. Bibcode:1991Natur.351..141S. doi:10.1038/351141a0.
- Kermack, D.W.; Kermack, K.A. (1984). "Dentitions, Tooth-Replacement and Jaw Articulation". The Evolution of Mammalian Characters. Springer US. pp. 66–68. doi:10.1007/978-1-4684-7817-4. ISBN 978-1-4684-7819-8.
- Kermack, K.A. (1956). "Tooth Replacement in Mammal-Like Reptiles of the Suborders Gorgonopsia and Therocephalia". Philosophical Transactions of the Royal Society B. 240 (670): 95–133. Bibcode:1956RSPTB.240...95K. doi:10.1098/rstb.1956.0013.
- Hopson, J.A. (1964). "Tooth replacement in cynodont, dicynodont, and therocephalian reptiles". Journal of Zoology. 142 (4): 625–654. doi:10.1111/j.1469-7998.1964.tb04632.x.
- Benoit, J.; Manger, P.R.; Rubidge, B.R. (2016). "Palaeoneurological clues to the evolution of defining mammalian soft tissue traits". Scientific Reports. 6: 25604. Bibcode:2016NatSR...625604B. doi:10.1038/srep25604. PMC 4860582. PMID 27157809.
- Boonstra L.D. 1934. "A contribution to the morphology of the mammal-like reptiles of the suborder Therocephalia". Annals of the South African Museum, 31: 215–267
- Ivakhnenko, M.F. (2008). "The First Whaitsiid (Therocephalia, Theromorpha)". Paleontological Journal. 42 (4): 409–413. doi:10.1134/S0031030108040102.
- Huttenlocker, A. (2009). "An investigation into the cladistic relationships and monophyly of therocephalian therapsids (Amniota: Synapsida)". Zoological Journal of the Linnean Society. 157 (4): 865–891. doi:10.1111/j.1096-3642.2009.00538.x.
- Rubidge, B.S.; Sidor, C.A. (2001). "Evolutionary Patterns Among Permo-Triassic Therapsids". Annual Review of Ecology and Systematics. 32: 449–480. doi:10.1146/annurev.ecolsys.32.081501.114113.
- Sigurdsen, T. (2006). "New features of the snout and orbit of a therocephalian therapsid from South Africa". Acta Palaeontologica Polonica. 51 (1): 63–75.
- Huttenlocker, A.K.; Sidor, C.A. (2016). "The first karenitid (Therapsida, Therocephalia) from the upper Permian of Gondwana and the biogeography of Permo-Triassic therocephalians". Journal of Vertebrate Paleontology. 36 (4): e1111897. doi:10.1080/02724634.2016.1111897.
- Nopcsa, F. (1933). "On the biology of the theromorphous reptile Euchambersia". Annals and Magazine of Natural History. 10. 12 (67): 125–126. doi:10.1080/00222933308673757.
- Watson, D.M.; Romer, A.S. (1956). "A classification of therapsid reptiles". Bulletin of the Museum of Comparative Zoology. 114: 35–89.
- Van Valen, L. (1960). "Therapsids as Mammals". Evolution. 14 (3): 304–313. doi:10.2307/2405973. JSTOR 2405973.
- Folinsbee, K.E.; Muller, J.; Reisz, R.R. (2007). "Canine Grooves: Morphology, Function, and Relevance to Venom". Journal of Vertebrate Paleontology. 27 (2): 547–551. doi:10.1671/0272-4634(2007)27[547:cgmfar]2.0.co;2. JSTOR 30126324.
- Sues, H.-D. (1996). "A reptilian tooth with apparent venom canals from the Chinle Group (Upper Triassic) of Arizona". Journal of Vertebrate Paleontology. 16 (3): 571–572. doi:10.1080/02724634.1996.10011340.
- Gong, E.; Martin, L.D.; Burnham, D.A.; Falk, A.R. (2009). "The birdlike raptor Sinornithosaurus was venomous". Proceedings of the National Academy of Sciences of the United States of America. 107 (2): 766–768. Bibcode:2010PNAS..107..766G. doi:10.1073/pnas.0912360107. PMC 2818910. PMID 20080749.
- Huttenlocker, A.K.; Sidor, C.A.; Angielczyk, K.D. (2015). "A new eutherocephalian (Therapsida, Therocephalia) from the upper Permian Madumabisa Mudstone Formation (Luangwa Basin) of Zambia". Journal of Vertebrate Paleontology. 35 (5): e969400. doi:10.1080/02724634.2015.969400.
- Mitchell, J.S.; Heckert, A.B.; Sues, H.-D. (2010). "Grooves to tubes: evolution of the venom delivery system in a Late Triassic "reptile"". Naturwissenschaften. 97 (12): 1117–1121. Bibcode:2010NW.....97.1117M. doi:10.1007/s00114-010-0729-0. PMID 21060984.
- Orr, C.M.; Delezene; Scott, J.E.; Tocheri, M.W.; Schwartz, G.T. (2007). "The comparative method and the inference of venom-delivery systems in fossil mammals". Journal of Vertebrate Paleontology. 27 (2): 541–546. doi:10.1671/0272-4634(2007)27[541:TCMATI]2.0.CO;2.
- Vaeth, R.H.; Rossman, D.A.; Shoop, W. (1985). "Observations of Tooth Surface Morphology in Snakes". Journal of Herpetology. 19 (1): 20–26. doi:10.2307/1564416. JSTOR 1564416.
- Bellairs, A.D'A. (1949). "Observations on the snout of Varanus, and a comparison with that of other lizards and snakes". Journal of Anatomy. 83 (2): 116–146. PMC 1273152. PMID 17105074.
- Abdel-Kader, T.G.; Ali, R.S.; Ibrahim, N.M. (2011). "The Cranial Nerves of Mabuya quinquetaeniata III: Nervus Trigeminus" (PDF). Life Science Journal. 8 (4): 650–669.
- Leitch, D.B.; Catania, K.C. (2012). "Structure, innervation and response properties of integumentary sensory organs in crocodilians". Journal of Experimental Biology. 215 (23): 4217–4230. doi:10.1242/jeb.076836. PMC 4074209. PMID 23136155.
- Bücherl, W. (1968). "Introduction". In Bücherl, W.; Buckley, E.E.; Deulofeu, V. (eds.). Venomous Animals and their Venoms. 1. New York: Academic Press. pp. 9–12. doi:10.1016/B978-1-4832-2949-2.50006-0. ISBN 9781483229492.
- Fry, B.G.; Wroe, S.; Teeuwisse, W.; van Osch, M.J.P.; Moreno, K.; Ingle, J.; McHenry, C.; Ferrara, T.; Clausen, P.; Scheib, H.; Winter, K.L.; Greisman, L.; Roelants, K.; van der Weerd, L.; Clemente, C.J.; Giannakis, E. (2009). "A central role for venom in predation by Varanus komodoensis (Komodo Dragon) and the extinct giant Varanus (Megalania) priscus". Proceedings of the National Academy of Sciences of the United States of America. 106 (22): 8969–8974. Bibcode:2009PNAS..106.8969F. doi:10.1073/pnas.0810883106. PMC 2690028. PMID 19451641.
- Ligabue-Braun, R.; Verli, H.; Carlini, C.R. (2012). "Venomous mammals: A review". Toxicon. 59 (7): 680–695. doi:10.1016/j.toxicon.2012.02.012. PMID 22410495.
- Weinstein, S.A.; Smith, T.L.; Kardong, K.V. (2009). "Reptile Venom Glands: Form, Function, and Future" (PDF). In Mackessy, S.P. (ed.). Handbook of Venoms and Toxins of Reptiles. Boca Raton: CRC Press. pp. 65–91.
- Goris, R.C. (2011). "Infrared Organs of Snakes: An Integral Part of Vision". Journal of Herpetology. 45 (1): 2–14. doi:10.1670/10-238.1.
- Krochmal, A.R.; Bakken, G.S.; LaDuc, T.J. (2004). "Heat in evolution's kitchen: evolutionary perspectives on the functions and origin of the facial pit of pitvipers (Viperidae: Crotalinae)". Journal of Experimental Biology. 207 (24): 4231–4238. doi:10.1242/jeb.01278. PMID 15531644.
- Benoit, J.; Abdala, F.; Manger, P.R.; Rubidge, B.S. (2016). "The Sixth Sense in Mammalian Forerunners: Variability of the Parietal Foramen and the Evolution of the Pineal Eye in South African Permo-Triassic Eutheriodont Therapsids". Acta Palaeontologica Polonica. 61 (4): 777–789. doi:10.4202/app.00219.2015.
- Anderson, J.M. (1977). "The microfloral succession: conclusions and discussion". A Review of Gondwana Permian Palynology with Particular Reference to the Northern Karoo Basin of South Africa. Memoirs of the Botanical Survey of South Africa. 41. pp. 42–58.