Gorgonopsia
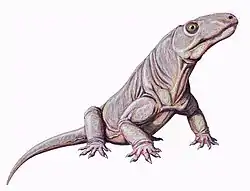
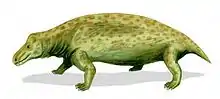
Gorgonopsia (from the Ancient Greek γοργών, meaning "gorgon", and ὄψ, meaning "aspect") is an extinct clade of sabre-toothed therapsids from the Middle to Upper Permian roughly 265 to 252 million years ago. They are characterised by a long and narrow skull, as well as elongated upper and sometimes lower canine teeth and incisors which were likely used as slashing and stabbing weapons. Postcanine teeth are generally reduced or absent. For hunting large prey, they possibly used a bite-and-retreat tactic, ambushing and taking a debilitating bite out of the target, and following it at a safe distance before its injuries exhausted it, whereupon the gorgonopsian would grapple the animal and deliver a killing bite. They would have had an incredible gape, possibly in excess of 90°, without having to unhinge the jaw.
| Gorgonopsians | |
|---|---|
 | |
| Skeleton of Inostrancevia alexandri at the Museo delle Scienze, Trento, Italy | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Therapsida |
| Clade: | Neotherapsida |
| Clade: | Theriodontia Seeley, 1895 |
| Clade: | †Gorgonopsia Lydekker, 1890 |
| Genera | |
They markedly increased in size as time went on, growing from small skull lengths of 10–15 cm (4–6 in) in the Middle Permian to bear-like proportions of up to 60 cm (2 ft) in the Upper Permian. The latest gorgonopsians, Rubidgeinae, were the most robust of the group and could produce especially powerful bites. They are thought to have been completely terrestrial and could walk with a semi-erect gait, with a similar terrestrial locomotory range as modern crocodilians. They may have been more agile than their prey items, but were probably inertial homeotherms rather than endotherms unlike contemporary therocephalians and cynodonts, and thus were probably comparatively less active. Though gorgonopsians were able to maintain a rather high body temperature, it is unclear if they would have also had fur, sweat glands, or whiskers. Their brains were reminiscent of modern reptilian brains, rather than those of living mammals. Most species may have been predominantly diurnal, active during the day, though some could have been crepuscular or nocturnal. They are thought to have had binocular vision, a parietal eye, a keen sense of smell, a functional vomeronasal organ ("Jacobson's organ"), and possibly a rudimentary eardrum.
The major therapsid groups had all evolved by 265 million years ago from a "pelycosaur" ancestor (a poorly defined group including all synapsids which are not therapsids). The therapsid takeover from pelycosaurs took place by the Middle Permian as the world progressively became drier. Gorgonopsians rose to become apex predators of their environments following the extinction of the dinocephalians and large therocephalians after the Middle Permian. Despite the existence of a single continent during the Permian, Pangaea, gorgonopsians have only been found in the Karoo Supergroup (primarily in South Africa, but also in Tanzania, Zambia, and Malawi), the Moradi Formation of Niger, and western Russia, with probable remains known from India. These places were semi-arid areas with highly seasonal rainfall. Gorgonopsian taxa vary very little, and consequently, many species have been named based on flimsy and highly variable differences since their discovery in the late 19th century, and the clade has been subject to several taxonomic revisions
They went extinct during a phase of the Permian–Triassic extinction event taking place at the very end of the Permian, in which major volcanic activity (which would produce the Siberian Traps) and resultant massive spike in greenhouse gases caused rapid aridification due to temperature spike, acid rain, frequent wildfires, and potential breakdown of the ozone layer. The large predatory niches would be taken over by the archosaurs (namely crocodilians and dinosaurs) in the Mesozoic.
Description

Earlier gorgonopsids in the Middle Permian were quite small, with skull lengths of 10–15 cm (4–6 in), whereas some later genera attained massive, bear-like sizes with skulls up to 60 cm (2 ft) in length. Nonetheless, small gorgonopsians remained abundant until extinction (though small species may actually represent juvenile specimens of other taxa).[1]
Like other Permian therapsids, gorgonopsians had developed several mammalian characteristics. These may have included a parasagittal gait (the limbs were vertically oriented and moved parallel to the spine) as opposed to the sprawling gait of amphibians and earlier synapsids. This gait change in therapsids may have been related to the reduction in tail size and phalangeal formula[2] (the number of bones per digit, which for gorgonopsians was 2.3.4.5.3 like reptiles[3]). Other developments included fibrous lamellar cortical bone, deeply-set teeth, and a secondary palate (which separates the mouth from the nasal cavity, but gorgonopsians may not have had this).[2]
Skull
Anatomy varies incredibly little between gorgonospians.[4] Many species are distinguished by vague proportional differences, and consequently smaller species may actually represent juveniles of larger taxa. Notably, the vomer at the tip of the snout varies among species in terms of the degree of its expansion, as well as the positions, degree of splay, and shape of the 3 ridges.[1] They typically feature a long and narrow skull.[3] Juvenile Rubidgea appear to have had snouts wider than long.[5] Unlike eutheriodonts, the occipital bone (at the back of the skull) is rectangular (wider than tall) and concave, as opposed to triangular.[6]:279

The gorgonopsian brain, like other non-mammaliaform therapsids, lacks an expansion of the neocortex, has a relatively large hindbrain compared to the forebrain, a large epyphysial nerve (found in creatures with a parietal eye on the top of the head), an enlarged pituitary gland, and an overall elongated shape; all-in-all resembling a reptilian brain.[7] The braincase was also rather reptilian, and is also comparatively smaller and not as thick as those of mammals.[6]:280 The flocculus, a lobe of the cerebellum, is proportionally large, and is related to the vestibulo–ocular reflex (which stablises gaze while moving the head). Judging by the orientation of the semi-circular canals in the ear (which have to be oriented parallel to the ground), the head of the gorgonopsian specimen GPIT/RE/7124 would have tilted forward by about 41°, increasing the overlap between the visual fields of the two eyes and improving binocular vision – useful to a predator. Unlike either reptiles or mammals, the semi-circular canals are flat, probably because they were wedged between the opisthotic (an inner ear bone) and supraoccipital bones.[7]
Teeth
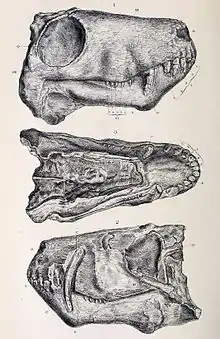
Like many mammals, gorgonopsians were heterodonts, with clearly defined incisors, canines, and postcanine teeth homologous with premolars and molars.[1] They had five incisors in the upper jaw (for most, the first three were the same size as each other, and the last two were shorter) and four on the bottom.[6]:17–18 In the majority of gorgonopsians, the incisors were large, and the upper canines were elongated into sabres, much like those of later sabre-toothed cats. Some gorgonopsians had exceptionally long upper canines, such as Inostrancevia, and some of them had a flange on the lower jaw to sheath the tip of the canine while the mouth was closed. Sabres are generally interpreted as having been used as stabbing or slashing weapons, which would have required an extremely wide gape. Both the upper and lower canines of Rubidgea were elongated, and the animal would have needed an even greater gape.[8] The serration pattern of gorgonopsians was most similar to those of theropod dinosaurs than to other synapsids.[9] The postcanine teeth were reduced in both size and number; many rubidgeines (the latest gorgonopsians) did not have postcanines in the lower jaw,[10] and Clelandia lacked them entirely.[5]
Gorgonopsians were polyphyodonts, and teeth grew continuously throughout an individual's life.[11] Like some therapsids, while there was one functional canine, another canine was growing to replace it when it inevitably broke off. The left and right sides of the jaws did not have to be synchronous, so, for example, the first canine on the left side could be functional while the first canine on the right side was still growing.[11] Such a method may have been in play so as to always have a set of functional canines, as having a single or no canines may have severely impeded hunting, and growing such large teeth may have taken a long time. On the other hand, because the functional canine is typically found in the front-most tooth socket (instead of equal occurrence in either socket), it is possible that canine replacement occurred a finite amount of times, and the animal would eventually be left with a single, permanent set of functional canines in these sockets. In 1984, British palaeontologists Doris and Kenneth Kermack suggested that the canines grew to match the size of the skull, and continually broke off until the animal stopped growing, and that gorgonopsians featured an early version of finite tooth replacement exhibited in many mammals. The tooth replacement patterns of the other teeth are unclear.[8] The postcanine teeth were replaced more slowly than the other teeth, likely due to their lack of functional significance.[1]
Postcranium

The seven cervical vertebrae (in the neck) are all the same size as each other except for the last one, which is shorter and lower; there is one atlas and one axis. Like sabre-toothed cats, the neck is long with well developed muscles, which would have been especially useful when the canines were sunk into an animal. Like other early synapsids, gorgonopsians have a single occipital condyle, and the articulation (the joints) of the cervical vertebrae is overall reptilian, permitting side-to-side movement of the head but restricting up-and-down motion. The last cervical is shaped more like the dorsal vertebrae.[6]:291–293
The dorsals are spool shaped and all appear about the same as each other. The spinous processes jut out steeply from the centra, and feature sharp keels on the front and back sides. Unlike eutheriodonts, gorgonopsians do not have distinguished lumbar vertebrae. Nonetheless, the dorsals equating to that series are similar to the lumbars of sabre-toothed cats with steeply oriented zygopophases, useful in stabalising the lower back especially when pinning down struggling prey.[6]:293–295
There are three sacral vertebrae, and the series attached to the pelvis by the first vertebra. The pelvis is reptilian, with separated ilium, ischium, and pubis. The femur is slightly s-shaped, and is short but longer and slenderer than the humerus. For most, the tibia and fibula strongly curve into each other, and the tibia is more robust than the fibula.[6]:295–299 The joint between the ankle and the heel bones may have been somewhat mobile. The fifth digit for both the hands and feet were not attached to the carpus/tarsus, and instead connected directly to the ulna/heel bone.[12]
Taxonomy
Classification
The first gorgonopsian remains were identified in the Beaufort Group of the Karoo Supergroup in a South African outcropping, and were classified as Gorgonops torvus in 1876 by English biologist Richard Owen. The name Gorgonops is derived from the Ancient Greek γοργών, meaning "gorgon", and ὄψ, meaning "aspect". Owen presumed that this and several other taxa he described from the supergroup were cold-blooded reptiles, yet they had teeth resembling those of carnivorous mammals, and so proposed classifying all of them under the newly coined order Theriodontia (which he placed in the class Reptilia). He decided to subdivide Theriodontia into families based on the anatomy of the nostrils (the bony narials)—"Mononarialia" for those with one opening in the skull for the nose as in mammals, "Binarialia" for those with two openings as in reptiles, and "Tectinarialia" for Gorgonops because its opening was overshadowed by a thick bone roof[13] (tectus is Latin for "covered, roofed, decked").[14] In 1890, English naturalist Richard Lydekker made Gorgonops the type species of the family Gorgonopsidae.[15] British palaeontologist Harry Seeley in 1895 believed Gorgonops lacked an opening in the temporal bone (temporal fenestra), which is a diagnostic feature of Theriodontia, and so elevated Gorgonopsidae to Gorgonopsia, distinct from Theriodontia. He classified all South African materials bearing both reptilian and mammalian traits into the order "Theriosuchia", and considered Gorgonopsia and Theriodontia suborders of it.[16] American palaeontologist Henry Fairfield Osborn completely reworked the classification of Reptilia in 1903, and erected two major groups: Diapsida and Synapsida,[17] and in 1905, South African palaeontologist Robert Broom created a third group, Therapsida, to house the "mammal-like reptiles", including Theriodontia. He also challenged Seeley's claim and relegated Gorgonops back to Theriodontia, but he placed it into his newly erected subgroup Therocephalia, dissolving Gorgonopsia.[18] In 1913, especially in light of an almost complete G. torvus skull discovered by the Reverend John H. Whaits, Broom reinstated Gorgonopsia.[19]
Gorgonopsians were first identified in Russia in the 1890s at the Sokolki locality on the Northern Dvina River in Siberia under the supervision of Russian palaeontologist Vladimir Prokhorovich Amalitskii. In a posthumous publication, it was described as Inostrancevia alexandri, and it is one of the best known and largest gorgonopsians. Since then, only a few more Russian genera have been described: Pravoslavlevia, Viatkogorgon, Suchogorgon, Leogorgon, and Nochnitsa.[20] In Africa, gorgonopsians have also been found in Karoo outcroppings in the Ruhuhu Valley, Tanzania; the Upper Luangwa Valley, Zambia; and Chiweta, Malawi.[6]:7 Gorgonopsians are conspicuously absent beyond these 2 areas.[20] In 1979, Chinese palaeontologist Yang Zhongjian described a Chinese gorgonopsian "Wangwusaurus tayuensis" based on teeth from the Late Permian Jiyuan Formation,[21] but in 1981, palaeontologists Denise Sigogneau-Russell and Ai-Lin Sun found the assigned material to be a random assemblage of which only two have even a remote similarity to Gorgonopsia.[22] In 2003, Indian palaeontologists Sanghamitra Ray and Saswati Bandyopadhyay assigned some skull fragments from the Late Permian Kundaram Formation to a medium-sized gorgonopsian,[23] though the gorgonopsian characteristics have also been documented in some therocephalians.[20] In 2008, a large and probably rubidgeine upper jaw fragment and canine was identified at the Late Permian Moradi Formation in Niger (one of the few low-latitude Late Permian tetrapod-bearing formations), and is the first evidence of a low-latitude gorgonopsian.[24]
The number of South African genera rapidly grew in the 20th-century, headed principally by Broom, whose extensive work on the Karoo therapsids—from the beginning of his career in the country in 1897 to his death in 1951—led to his description of 57 gorgonopsian holotype specimens and 29 genera. Many of Broom's taxa would later be invalidated.[25] Many other contemporary workers created wholly new species or genera based on single specimens.[6]:57 Consequently, Gorgonopsia has been the subject of much taxonomic turmoil, and is one of the most problematic synapsid groups. Because the skull anatomy differs very little across taxa, many are defined based on vague proportional differences, including even the well-known members. Nominal species are distinguished predominantly by traits which are known to be quite variable depending on the age of the individual, including eye orbit size, snout length, and number of postcanine teeth. Thus, it is possible that some taxa are synonymous with each other, and represent different stages of development.[26]
Among the first attempts to organise the clade was carried out by British zoologist David Meredith Seares Watson and American palaeontologist Alfred Romer in 1956, who split it into twenty families, of which the members of three (Burnetiidae, Hipposauridae, and Phthinosuchidae) are not considered gorgonopsians anymore.[5] In 1970 and again in 1989, predominantly considering African taxa, Sigogneau-Russell published a comprehensive monograph on Gorgonopsia (defining it as an infraorder), and recognised only two families: Watongiidae and Gorgonopidae. Watongia was moved to Varanopidae in 2004. She split Gorgonopidae into three subfamilies—Gorgonopsinae, Rubidgeinae, and Inostranceviinae—and reduced the number of genera to twenty-three.[27] In 2002, Russian palaeontologist Mikhail Feodosʹevich Ivakhnenko, considering the Russian taxa, instead considered Gorgonopsia a suborder, and grouped it together with Dinocephalia into the order "Gorgodontia". He divided Gorgonopsia into the superfamilies "Gorgonopioidea" (families Gorgonopidae, Cyonosauridae, and Galesuchidae) and "Rubidgeoidea" (Rubidgeidae, Phtinosuchidae, and Inostranceviidae). In 2007, biologist Eva V. I. Gebauer, in her comprehensive review of Gorgonopsia (her PhD dissertation), rejected Ivakhnenko's model in favour of Sigogneau-Russell's,[6]:57 and further reduced the number of genera to fourteen in addition to the Russian genera: Aloposaurus, Cyonosaurus, Aelurosaurus, Sauroctonus, Scylacognathus, Eoarctops, Gorgonops, Njalila, Lycaenops, Arctognathus, Aelurognathus, Sycosaurus, Clelandina, and Rubidgea.[6]:244 In general, Sigogneau-Russell's model is supported, but there is little consensus on which genera can be assigned to which subfamilies.[5] In 2015, American palaeontologist Christian F. Kammerer and colleagues redescribed Eriphostoma (which was labelled as an indeterminate theriodont) as a gorgonopsian,[28] and sunk Scylacognathus and the next year Eoarctops into it.[5]
The first phylogeny (family tree) of the members of Gorgonopsia was published in 2016 by American palaeontologist Christian F. Kammerer, who specifically investigated Rubidgeinae, and re-described both the subfamily and the nine species he assigned to it (reducing the number from thirty-six species). Kammerer also resurrected Dinogorgon, Leontosaurus, Ruhuhucerberus, and Smilesaurus. Kammerer was unsure if Leontosaurus, Clelandina, Dinogorgon, and Rubidgea all represent the same taxon or not (for which Dinogorgon has priority), but he decided to classify all of them in the tribe Rubidgeini pending further examination.[5] In 2018, Kammerer and Russian palaeontologist Vladimir Masyutin identified a new genus Nochnitsa as the basalmost known gorgonopsians, and found that all Russian taxa (except Viatkogorgon, which is in the outclade) form a completely separate clade from the African taxa.[20] Also in 2018, palaeobiologist Eva-Maria Bendel, Kammerer, and colleagues resurrected Cynariops.[1]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phylogeny of Gorgonopsia according to Bendel et al. 2018[1] |
Evolution
Synapsida has traditionally been split into the basal "Pelycosauria" and the derived Therapsida. The former comprises cold-blooded creatures with a sprawling gait and presumably lower metabolism which evolved in the Upper Carboniferous. Through the middle to late 20th-century, American palaeontologist Everett C. Olson investigated synapsid diversity in the Middle Permian San Angelo, Flowerpot, and Chickasha Formations in North America, and noted that pelycosaur diversity reduced from six to three in these formations, and that they coexisted with several fragmentary specimens which he interpreted as therapsids. He then suggested the shift took place during the Middle Permian (Olson's Extinction); however, the classification of those "therapsids" and the age of the formations have since been challenged. Thus, the exact timing of the adaptive shift from pelycosaur-grade to therapsid-grade is unclear, but the seven major therapsid groups (Biarmosuchia, Dinocephalia, Anomodontia, Gorgonopsia, Therocephalia, and Cynodontia) had existed by 265 million years ago.[29]
| Synapsida[29] | |||||||||||||||||||||||||||||||||||||||
|

The Permian progressively became dryer and dryer. In the Upper Carboniferous and Lower Permian, pelycosaurs seem to have clung to the everwet coal swamp habitats near the equator (fossils known within 10° of either side of the palaeoequator); beyond this to about 30° was an expansive desert which extended all the way to the coast, separating the swamps from the temperate regions. By the Middle Permian, the equatorial forests had switched to a seasonal wet/dry system, but the swamps were connected to the temperate zones via coastal passages along East Pangaea, allowing cross-continental migration from what is now South Africa to what is now Russia. Therapsids appear to have evolved in this seasonally humid/dry landscape, expanding even into the temperate zones. At this point, synapsids were the only large terrestrial animals of their environment; and pelycosaurs may not have been able to adapt to the aridification. At about the time of pelycosaur extinction, therapsids experienced a major adaptive radiation (all carnivores) continuing into the Upper Permian.[29]
Throughout the Middle Permian, the often gigantic dinocephalians were the dominant animals of their ecosystems. They disappear from the fossil record during the Wordian, just before the Capitanian mass extinction event caused by volcanic activity which has formed the Chinese Emeishan Traps. The exact cause of their extinction is unclear, but they were replaced by gorgonopsians and dicynodonts (which began to greatly increase in size) and the smaller therocephalians.[30] The rubidgeans were the latest, and consequently the most massive and heavily built, gorgonopsian group.[5]
Palaeobiology
Bite

Gorgonopsians were likely active predators. The rubidgeines have an especially built skull among gorgonopsians, comparable to those of enormous macropredators which use their skulls as their primary weapon, such as mosasaurs or some theropod dinosaurs. Less robust gorgonopsians with longer canines and much weaker bite, such as Smilesaurus or Inostrancevia, instead probably used their canines for slashing, much more similar to sabre-toothed cats. The postcanines of Clelandina were replaced by a smooth ridge unlike dicynodonts which have a blade-like keratinous ridge, and it may have predominantly gone after prey it could swallow whole. Gorgonopsian taxa did coexist with each other—as many as seven at one time–and the fact that some rubidgeines possess postcanines while some other contemporary ones do not suggests that they practiced niche partitioning and pursued different prey items.[5]
The elongated canines have generally been thought to have been instrumental in their hunting tactics. The gorgonopsian jaw hinge was double jointed and made up of somewhat mobile and rotatable bones, which would have allowed them to open their mouths incredibly wide–perhaps in excess of 90°–without having to unhinge the jaw.[31] It has alternatively been suggested that sabres among any sabre-toothed synapsid group evolved primarily due to sexual selection (as a form of mating display) as in some modern deer species, but this is difficult to test given the lack of living sabre-toothed synapsid predators. In sabre-toothed cats, long-sabred ("dirk-toothed") taxa are thought to have been pursuit hunters, whereas short-toothed ("scimitar-toothed") taxa are thought to have been ambush predators.[32] Among the dirk-toothed cats, these predators are suggested to have killed with a well-placed slash to the throat after grappling prey, but gorgonopsians may have been less precise with bite placement, armed with reptilian jaws and tooth arrangements. Instead, gorgonopsians possibly used a bite-and-retreat tactic: the predator would ambush its quarry and take a sizable and debilitating bite out of it, and then follow as the prey tried to escape before succumbing to its injury, whereupon the gorgonopsian would deliver a killing bite. Because the postcanines were reduced or entirely absent, meat would have been forcefully torn away from a carcass and swallowed whole.[31] This "puncture–pull" strategy is also hypothesised to have been done by theropod dinosaurs.[9]

Gorgonopsians, along with other early carnivores as well as crocodiles, predominantly relied on "Kinetic-Inertial system" (KI) of biting down onto prey, in which the pterygoid and temporalis muscles rapidly clamped the jaws shut, using momentum and the kinetic energy of the jaws and teeth to grapple the victim. Mammalian carnivores, including sabre-toothed cats, instead rely mainly on the "Static-Pressure system" (SP) where the temporalis and masseter muscles produce a strong bite force to kill prey.[10] The temporalis and masseter had only separated in mammals, and gorgonopsians instead had a muscle stretching from the underside of the skull roof, back to the squamosal bone (at the back of the skull), and across the cheekbones. The part anchored by the cheeks stabalised the jawbone and allowed it to move side-to-side while closing. This may have been very important in biting, as the cheekbones get stronger in tandem with the canines getting longer.[6]:278
Smaller gorgonopsians, such as Cyonosaurus (which may actually represent a juvenile of a different species), had gracile skulls and sabres, and may have acted much like jackals and foxes. Bigger gorgonopsians, such as Gorgonops, had long robust snouts with strongly flared cheeks, which would have supported strong pterygoids and a powerful KI bite. The medium-size Arctognathus had a box-like skull and resultantly powerful snout, which would have allowed strong bending and torsion movements, and a combination of both KI and SP bite elements. Even bigger gorgonopsians, such as Arctops, had a shorter and more convex snout like the earlier sphenecodont Dimetrodon, and would have been able to rapidly clamp the jaws shut from a wide gape (which would have been necessary given the long canines). The even larger Rubidgeinae had extremely powerful, heavily built, buttressed skulls, with wide snouts, strongly flanged cheeks, and exceedingly long teeth; the sabres of Rubidgea atrox are longer than the teeth of Tyrannosaurus.[10]
Unlike mammalian carnivores, gorgonopsians (and therocephalians) had reduced or completely lacked postcanines, and the jaw likely could not exert shearing pressure necessary for crushing bones open to access the bone marrow. They had no use for this since synapsids at this time did not have bone marrow.[33]
Locomotion

Gorgonopsians are considered to have been strictly terrestrial.[34] They are thought to have been able to move with an erect gait similar to that used by crocodilians, the limbs positioned almost vertically as opposed to horizontally as in the sprawling gait of lizards. The glenoid cavity on the shoulder blade is strongly angled tailwards, so the limbs had limited forward movement, and they may have had a short stride length. Lizards often move their spines side to side to increase stride length, but the more vertically orientated facet joints connecting the vertebrae in gorgonopsians would have made the spine more rigid and stable, encumbering such movement.[6]:259–260
The gorgonopsian shoulder joint has a highly unusual configuration. The humeral head which connects to the shoulder is longer than the glenoid, so it could not fit into the cavity. Consequently, they may have been attached with a large mass of cartilage, with the humerus performing a rolling movement over the glenoid. This could theoretically make the angle between the humerus and the glenoid anywhere from 80–145° when facing the animal. If the angle was on the lower end (i.e., angled slightly headwards), this would have been a rather firm joint, allowing the deltoids to exert great force through the forelimb, such as when pinning down struggling prey, or holding down a carcass while ripping off flesh. If the humerus was positioned at a higher angle (angled downwards), this could have permitted enhanced extension forwards and backwards (along the long axis) and thus greater stride length, useful in an attack or short chases. The shoulder blade expands off to the sides of the animal (protrudes laterally), also providing a large attachment for the deltoids. All the scapulohumeral muscles had strongly developed attachments, particularly the deltoids. When extending the forelimbs, the deltoids may have raised the front side (anterior margin) of the humerus, and coracobrachialis muscle lowered the back side (posterior margin). When retracting the forelimb, the pectoralis muscle may have pushed the anterior margin down, and the subscapularis muscle pulled the posterior margin up.[6]:260–264
The pelvis joint has the usual ball-and-socket joint configuration. The somewhat flattened femoral head could theoretically have fit into the hip socket at a wide range of angles. In 1982, palaeontologist Tom S. Kemp suggested that early theriodonts, including gorgonopsians, could place the femur at both a horizontal angle in a sprawling gait, as well as a more vertical angle in an erect gait. He compared the locomotory habits of these creatures to those of crocodilians, which utilise a sprawling gait over short distances, but switch to an erect one while running or moving over longer distances. Though the hip of Sauroctonus seems to be anatomically intermediate between Dimetrodon and mammals—with the ilium expanded more in the headwards direction than the tailwards, and the pubis somewhat reduced—the puboischiofemoralis muscle (a large muscle carried only by reptiles which runs from the pelvis to the femur) extensively attached to the underside of the pubis and ischium, which would have allowed it to produce a strong adducting force (drawing the legs closer to the body), useful in a sprawling gait. It is also conceivable that gorgonopsians primarily engaged this muscle while grappling struggling prey.[6]:264–270 The shins are relatively short compared to the femur, which suggests gorgonopsians were not well adapted for running long distances.[6]:298–299

In regard to how the feet were placed on the ground, gorgonopsians are the only early therapsids which present ectataxony (the last digit bears the most weight), homopody (fooprints and handprints look the same), and semi-plantigrady (to some degree, the feet were placed flat on the ground).[35] These adaptations may have made gorgonopsians swifter and more agile than their prey.[31] Gorgonopsians had rather nimble digits, indicative of grasping capability for both the hands and feet, possibly for grappling struggling prey to prevent excessive load bearing on, and consequential fracturing or breaking of, the canines while they were sunk into the victim.[12]
Senses
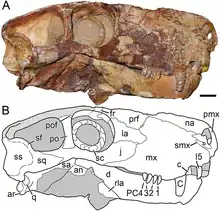
Unlike eutheriodonts, but like some ectothermic creatures today, all gorgonopsians possessed a pineal eye on the top of the head, which is used to detect daylight (and thus, the optimal temperature to be active). It is possible that other theriodonts lost this due to the evolution of either endothermy, intrinsically photosensitive retinal ganglion cells in the eyes—in tandem with the loss of colour vision and a shift to nocturnal life–or both.[36] Nocturnal behaviour has long been assumed to have originated in mammals (nocturnal bottleneck), but the large orbit size and presence of sclerotic rings in many early synapsids, stretching as far back as the Carboniferous, would suggest that the ability to venture out in low-light conditions evolved much earlier. Based on these aspects, Sauroctonus parringtoni may have had mesopic vision, and Cyonosaurus scotopic or photopic vision.[37] The diameters of the sclerotic rings for the small Viatkogorgon are proportionally large, with an inner diameter of 1.5 cm (0.59 in) and outer diameter of 2.3 cm (0.91 in), compared to a diameter of 2.8 cm (1.1 in) for the orbit itself, which suggests it made predominantly nocturnal excursions.[38] Among gorgonopsians, the rubidgeine Clelandina has unusually small sclerotic rings, indicating it had photopic vision and was strictly diurnal; Kammerer suggested that niche partitioning among rubidgeines (as there have been as many as seven different taxa coexisting in an area), in part, took the form of different species being active at different times of the day, but the sclerotic rings of only Clelandina among this subfamily have been identified, making this hypothesis highly speculative.[5]
Gorgonopsians have a rather short nasal cavity, like pelycosaurs, but it features abundant longitudinal ridges behind the internal nostrils (which connect the nasal cavity to the throat); because respired air would not have passed through them, these are typically interpreted as having been olfactory turbinates, and would have given gorgonopsians a rather highly developed sense of smell.[39] Gorgonopsians possessed a vomeronasal organ ("Jacobson's organ")—a part of the accessory olfactory system–which would have been placed at the base of the nasal septum; unlike dicynodonts and therocephalians, there seems to have been a canal connecting the organ with the mouth, indicating it was functional in gorgonopsians.[40]
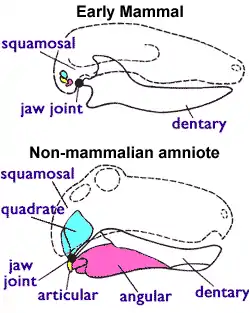
It has been suggested that early theriodonts (including gorgonopsians) possessed an eardrum, unlike earlier pelycosaurs, because the connection between the quadrate bone (at the jaw hinge) and the pterygoid bone (at the palate) was beginning to reduce, allowing the quadrate to independently vibrate to a degree. This may have allowed the detection of air-borne sounds with a low amplitude of less than 1 mm (0.039 in), but the eardrum would have been supported by cartilage or ligaments instead of bone. If correct, then the postdentary bones (which in early mammals form the middle ear bones) would have needed to become detached from the dentary (jawbone); the gorgonopsian fossil record seems to indicate the postdentary-dentary connection was reduced. For gorgonopsians, it has alternatively been suggested that the isolation of the quadrate was to cause streptostyly (rotatable quadrate) to increase the gape rather than to facilitate hearing.[2]
Thermoregulation
A major anatomical shift occurred between earlier pelycosaurs and therapsids, which is postulated to have been related to an increasing metabolism and the origins of homeothermy (maintenance of a high body temperature). The evolution of a secondary palate, and the separation of the mouth from the nasal cavity, may have increased ventilation efficiency associated with high levels of aerobic activity; gorgonopsians did not have a bony secondary palate, but possibly had one of soft tissue, but even then, it is also possible it was mainly for eating behaviour instead. The reorganisation of the skeleton (from a sprawling to a parasagittal gait) has been postulated to be indicative of the presence of a diaphragm, and thus also enhanced ventilation for aerobic activity; but it could have instead been to increase acceleration or agility, which does not necessarily equate to intense aerobic activity, much like in crocodiles. Fibrous lamellar cortical bone, which all early therapsids had, would indicate an increased growth rate, but this may not be linked to metabolic rate.[41]
Modern large reptiles naturally give off body heat at a slower rate than smaller ones, and are considered "inertial homeotherms", but they maintain a low body temperature of 25–30 °C (77–86 °F). If therapsids required a higher body temperature of 35–40 °C (95–104 °F), they would either have needed to have been endotherms (generating their own body heat) or have had greater control over heat loss (that is, better homeothermy). The parasagittal gait may have aided the latter, as it would have kept most of the body off the ground as well as allowed blood to stay in the abdomen instead of having to circulate through the appendages, both of which would reduce heat transfer to the ground and stabalise core temperature. The reduced tail would have also reduced the total surface area of the animal, further minimising heat loss.[42] Among therapsids, only eutheriodonts (not gorgonopsians) have respiratory nasal turbinates, which help retain moisture while breathing in large quantities of air, and its evolution is typically associated with the beginning of "mammalian" oxygen consumption rates and the origins of endothermy.[39]
If gorgonopsians were inertial homeotherms, it is not impossible that they had hair. The snout is typically riddled with foramina (small holes which confer with blood vessels), which could potentially point to the existence of loose skin (as opposed to scales), hair, various skin glands (such as sweat glands), and whiskers; however, some reptiles present a similar patterning of foramina, which are instead related to dental development rather than skin.[43]
Palaeopathology
The anterolateral aspect of the left radius (a forearm bone) of the gorgonopsian specimen NHCC LB396 presents a circular bony lesion, featuring irregular-to-radial spikes made of cortical bone surrounded by a thin layer of subperiosteal bone, which grew rapidly over a single growing season. This is consistent with periostitis most likely stemming from subperiosteal haematoma. This specific condition as well as the fast growth rate are more reminiscent of mammals and dinosaurs than crocodilians or monitor lizards. Among early synapsids, the only other pathology noted is osteomyelitis in several pelycosaur groups.[44]
The labial (tongue) side of the tooth root of a functional canine of RB382 presents as many as 8 lesions, clustering along the midline of the tooth, which resemble miniature teeth with a pulp, dentine, and a thin enamel coating. They are roughly circular—with diameters varying from 0.3–3.9 mm (0.012–0.154 in)–though they become less circular at around the middle point of the root until passing the cervix of the tooth. This is roughly consistent with the human ailment odontoma, the most frequent type of odontogenic tumour, which previously only extended a few million years back in the fossil record. At 255 million years old, RB382 presents the oldest known case of odontoma.[45]
Palaeoecology
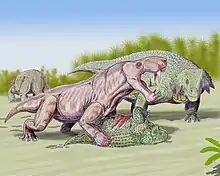
Following the extinction of the dinocephalians and (in South Africa) the basal therocephalians Scylacosauridae and Lycosuchidae, gorgonopsians evolved from small and uncommon forms into large apex predators. Through the Middle to Upper Permian, in South Africa the dicynodonts were the most common animals, whereas the pareiasaurs Deltavjatia and Scutosaurus were the most abundant in the gorgonopsian-bearing Russian formations.[46] During the Upper Permian, the South African Beaufort Group was a semi-arid cold steppe featuring large, seasonal (ephemeral) rivers and floodplains draining water sources much farther north into the Karoo Sea, with some occurrences of flash floods after sudden, heavy rainfall;[47][48] the distribution of carbonates is consistent with present-day caliche deposits which form in climates with an average temperature of 16–20 °C (61–68 °F) and 100–500 mm (3.9–19.7 in) of seasonal rainfall.[48] The Salarevskian Formation in western Russia was also probably deposited in a semi-arid environment with highly seasonal rainfall, and featured hygrophyte and halophyte plants in coastal areas, as well as more drought-resistant conifers at higher elevations.[49] The Moradi Formation was an arid desert, primarily dominated by the reptile Moradisaurus and the pareiasaur Bunostegos, which likely fed on conifers.[24]
Gorgonopsians went extinct at the end of the Upper Permian during the Permian-Triassic extinction event, which was primarily caused by volcanism which formed the Siberian Traps. The resultant massive spike in greenhouse gases caused rapid aridification due to: temperature spike (as much as 8–10°C at the equator, with average equatorial temperatures of 32–35 °C, or 90–95 °F, at the beginning of the Triassic), acid rain (with pH as low as 2 or 3 during eruption and 4 globally, and the subsequent dearth of forests for the first 10 million years of the Triassic), frequent wildfires (though they were already rather common throughout the Permian), and potential breakdown of the ozone layer (possibly briefly increasing UV radiation bombardment by 400% at the equator and 5000% at the poles).[50] Among therapsids, small therocephalians and large herbivorous anomodonts managed to cross the Permian–Triassic boundary, and survived respectively until the Middle and Upper Triassic, but only small-bodied species of cynodonts survived into the Jurassic, whose descendants would include mammals. The niches gorgonopsians left open were eventually filled by the archosaurs (including crocodiles and dinosaurs) during the early stages of the Triassic.[51]
Notes
- ce, cerebellum; cnI, olfactory nerve; cnV +vcm—trigeminal nerve and vena capitis medialis; cnVI, abducens nerve; cnVII, facial nerve; cnIX-XI, glossopharyngeal and vagoaccessory nerves; cnXII, hypoglossal nerve; en, epiphyseal nerve; fb, forebrain; fcl, flocculus; ibic, internal branch of the internal carotid; lob, left olfactory bulb; ob, olfactory bulb; ot, olfactory tract; pg, pituitary gland; pgll, pituitary gland lateral lobes; pf, pontine flexure; rob, right olfactory bulb; vc, vidian canal; vc=spa, vidian canal where the sphenopalatine artery passes; vcd, vena capitis dorsalis
References
- Bendel, Eva-Maria; Kammerer, Christian F.; Kardjilov, Nikolay; Fernandez, Vincent; Fröbisch, Jörg (2018). "Cranial anatomy of the gorgonopsian Cynariops robustus based on CT-reconstruction". PLOS ONE. 13 (11): e0207367. doi:10.1371/journal.pone.0207367. PMC 6261584. PMID 30485338.
- Laurin, M. (1998). "New data on the cranial anatomy of Lycaenops (Synapsida, Gorgonopsidae), and reflections on the possible presence of streptostyly in gorgonopsians". Journal of Vertebrate Paleontology. 18 (4): 765–776. doi:10.1080/02724634.1998.10011105.
- Swinton, W. E. (1954). Fossil amphibians and reptiles. British Museum (Natural History). p. 33.
- Angielczyk, Kenneth D.; Kammerer, Christian F. (2018). "Non-Mammalian synapsids: the deep roots of the mammalian family tree". In Zachos, Frank E.; Asher, Robert J. (eds.). Mammalian Evolution, Diversity and Systematics. Berlin: De Gruyter. ISBN 9783110275902.
- Kammerer, Christian F. (2016). "Systematics of the Rubidgeinae (Therapsida: Gorgonopsia)". PeerJ. 4: e1608. doi:10.7717/peerj.1608. PMC 4730894. PMID 26823998.
- Gebauer, E. V. I. (2007). Phylogeny and Evolution of the Gorgonopsia with a Special Reference to the Skull and Skeleton of GPIT/RE/7113 (PhD). Eberhard-Karls University of Tübingen.
- Araújo, Ricardo; Fernandez, Vincent; Polcyn, Michael J.; Fröbisch, Jörg; Martins, Rui M. S. (2017). "Aspects of gorgonopsian paleobiology and evolution: insights from the basicranium, occiput, osseous labyrinth, vasculature, and neuroanatomy". PeerJ. 5: e3119. doi:10.7717/peerj.3119. PMC 5390774. PMID 28413721.
- Kermack, D. M.; Kermack, K. A. (1984). "Dentitions, Tooth-Replacement and Jaw Articulation". The Evolution of Mammalian Characters. pp. 71–79. doi:10.1007/978-1-4684-7817-4_5. ISBN 978-1-4684-7817-4.
- Whitney, M. R.; LeBlanc, A. R. H.; Reynolds, A. R.; Brink, K. S. (2020). "Convergent dental adaptations in the serrations of hypercarnivorous synapsids and dinosaurs". Biology Letters. 16 (12). doi:10.1098/rsbl.2020.0750.
- Van Valkenburgh, B.; Jenkins, I. (2002). "Evolutionary Patterns in the History of Permo-Triassic and Cenozoic Synapsid Predators". The Paleontological Society Papers. 8: 273–275. doi:10.1017/S1089332600001121.
- Kermack, K. A. (1956). "Tooth replacement in mammal-like reptiles of the suborders Gorgonopsia and Therocephalia". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 240 (670): 95–133. doi:10.1098/rstb.1956.0013.
- Kümmell, S. B.; Frey, E. (2014). "Range of Movement in Ray I of Manus and Pes and the Prehensility of the Autopodia in the Early Permian to Late Cretaceous Non-Anomodont Synapsida". PLOS ONE. 9 (12): e113911. doi:10.1371/journal.pone.0113911. PMC 4269487. PMID 25517726.
- Owen, R. (1986). Descriptive and illustrated catalogue of the fossil Reptilia of South Africa in the collection of the British museum. British Museum (Natural History). pp. 27–29.
- tectus in Charlton T. Lewis (1891) An Elementary Latin Dictionary, Harper & Brothers
- Lydekker, R. (1890). Catalogue of the fossil Reptilia and Amphibia in the British Museum (Natural history) Part IV. British Museum (Natural History). p. 111.
- Seeley, H. G. (1895). "Researches on the structure, organization, and classification of the fossil reptilia.—Part IX. section 1. On the Therosuchia". Annals and Magazine of Natural History. 13 (6): 375. doi:10.1080/00222939408677718.
- Osborn, H. F. (1903). "The Reptilian Subclasses Diapsida and Synapsida and the Early History of the Diaptosauria. Volume I. Part VIII" (PDF): 451–466. Cite journal requires
|journal=(help) - Broom, R. (1905). "On the use of the term anomodont". Records of the Albany Museum. 1: 266–269.
- Broom, R. (1913). "On the Gorgonopsia, a Suborder of the Mammal-like Reptiles". Proceedings of the Zoological Society of London. 1913 (2): 225–230. doi:10.1111/j.1469-7998.1913.tb07574.x.
- Kammerer, C. F.; Masyutin, V. (2018). "Gorgonopsian therapsids (Nochnitsa gen. nov. and Viatkogorgon) from the Permian Kotelnich locality of Russia". PeerJ. 6: e4954. doi:10.7717/peerj.4954. PMC 5995105. PMID 29900078.
- Young, C. C. (1979). "A Late Permian fauna from Jiyuan, Henan". Vertebrata PalAsiatica. 17 (2): 99–113.
- Sigogneau-Russell, D.; Sun, A.-L. (1981). "A brief review of Chinese synapsids". Geobios. 14 (2): 276. doi:10.1016/S0016-6995(81)80012-5.
- "Late Permian vertebrate community of the Pranhita–Godavari valley, India". Cite journal requires
|journal=(help) - Smiley, T. M.; Sidor, C. A.; Maga, A.; Ide, O. (2008). "The vertebrate fauna of the Upper Permian of Niger. VI. First evidence of a gorgonopsian therapsid". Journal of Vertebrate Paleontology. 28 (2): 543–547. doi:10.1671/0272-4634(2008)28[543:TVFOTU]2.0.CO;2.
- Wyllie, A. (2003). "A review of Robert Broom's therapsid holotypes: Have they survived the test of time?" (PDF). Palaeontologia Africana. 39 (39): 2.
- Kammerer, C. F. (2013). "Theriodontia: Introduction". Early Evolutionary History of the Synapsida. Vertebrate Paleobiology and Paleoanthropology. Springer. pp. 165–169. doi:10.1007/978-94-007-6841-3_10. ISBN 978-94-007-6841-3.
- Sigogneau-Russell, Denise (1989). Wellnhofer, Peter (ed.). Theriodontia I: Phthinosuchia, Biarmosuchia, Eotitanosuchia, Gorgonopsia. Encyclopedia of Paleoherpetology. 17 B/I. Stuttgart: Gustav Fischer Verlag. ISBN 978-3437304873.
- Kammerer, C. F.; Smith, R. M. H.; Day, M. O.; Rubidge, B. S. (2015). "New information on the morphology and stratigraphic range of the mid‐Permian gorgonopsian Eriphostoma microdon Broom, 1911". Papers in Palaeontology. 1 (2): 201–221. doi:10.1002/spp2.1012.
- Kemp, T. S. (2006). "The origin and early radiation of the therapsid mammal‐like reptiles: a palaeobiological hypothesis". Journal of Evolutionary Biology. 19 (4): 1231–1247. doi:10.1111/j.1420-9101.2005.01076.x. PMID 16780524. S2CID 3184629.
- Day, M. O.; Ramezani, J.; Bowring, S. A.; et al. (2015). "When and how did the terrestrial mid-Permian mass extinction occur? Evidence from the tetrapod record of the Karoo Basin, South Africa". Proceedings of the Royal Society B. 282 (1811): 20150834. doi:10.1098/rspb.2015.0834. PMC 4528552. PMID 26156768.
- Antón, Mauricio (2013). Sabertooth. Bloomington, Indiana: University of Indiana Press. pp. 204–207. ISBN 9780253010421.
- Randau, M.; Carbone, C.; Turvey, S. T. (2013). "Canine Evolution in Sabretoothed Carnivores: Natural Selection or Sexual Selection?". PLOS ONE. 8 (8): e72868. doi:10.1371/journal.pone.0072868. PMC 3738559. PMID 23951334.
- Cruikshank, A. R. I. (1973). "The Mode of Life of Gorgonopsians" (PDF). Palaeontologica Africana. 15 (1–2): 65–67.
- Kriloff, A.; Germain, D.; Canoville, A.; Vincent, P.; Sache, M.; Laurin, M. (2008). "Evolution of bone microanatomy of the tetrapod tibia and its use in palaeobiological inference". Journal of Evolutionary Biology. 21 (3): 807–826. doi:10.1111/j.1420-9101.2008.01512.x. PMID 18312321.
- Marchetti, L.; Klein, H.; Buchwitz, M.; et al. (2019). "Permian-Triassic vertebrate footprints from South Africa: Ichnotaxonomy, producers and biostratigraphy through two major faunal crises". Gondwana Research. 72: 159. doi:10.1016/j.gr.2019.03.009.
- Benoit, J.; Abdala, F.; Manger, P. R.; Rubidge, B. S. (2016). "The Sixth Sense in Mammalian Forerunners: Variability of the Parietal Foramen and the Evolution of the Pineal Eye in South African Permo-Triassic Eutheriodont Therapsids". Acta Palaeontologica Polonica. 61 (4): 777–789. doi:10.4202/app.00219.2015. S2CID 59143925.
- Angielczyk, K. D.; Schmitz, L. (2014). "Nocturnality in synapsids predates the origin of mammals by over 100 million years". Proceedings of the Royal Society B. 281 (1793). doi:10.1098/rspb.2014.1642. PMC 4173690. PMID 25186003.
- Kammerer, Christian F.; Masyutin, Vladimir (2018). "Gorgonopsian therapsids (Nochnitsa gen. nov. and Viatkogorgon) from the Permian Kotelnich locality of Russia". PeerJ. 6: e4954. doi:10.7717/peerj.4954. PMC 5995105. PMID 29900078.
- Hillenius, W. J. (1992). Nasal turbinates and the evolution of mammalian endothermy (PhD). Oregon State University. pp. 74–76. hdl:1957/35986.
- Hillenius, W. J. (2000). "Septomaxilla of nonmammalian synapsids: Soft‐tissue correlates and a new functional interpretation". Journal of Morphology. 245 (1): 29–50. doi:10.1002/1097-4687(200007)245:1<29::AID-JMOR3>3.0.CO;2-B.
- Kemp, T. S. (2006). "The origin of mammalian endothermy: a paradigm for the evolution of complex biological structure". Zoological Journal of the Linnean Society. 147 (4): 473–488. doi:10.1111/j.1096-3642.2006.00226.x.
- Turner, J. S.; Tracey, C. R. (1986). "Body Size, Homeothermy and the Control of Body Heat in Mammal-like Reptiles". The Ecology and Biology of Mammal-like reptiles (PDF). Smithsonian Institution Press.
- Van Velan, L. (1960). "Therapsids as Mammals". Evolution. 14 (3): 304–313. doi:10.2307/2405973. JSTOR 2405973.
- Kato, K. M.; Rega, E. A.; Sidor, C. A.; Huttenlocker, A. K. (2020). "Investigation of a bone lesion in a gorgonopsian (Synapsida) from the Permian of Zambia and periosteal reactions in fossil non-mammalian tetrapods". Philosophical Transactions of the Royal Society B. 375 (1793). doi:10.1098/rstb.2019.0144. PMC 7017433. PMID 31928188.
- Whitney, M. R.; Mose, L.; Sidor, C. A. (2017). "Odontoma in a 255-Million-Year-Old Mammalian Forebear". JAMA Oncology. 3 (7): 998–1000. doi:10.1001/jamaoncol.2016.5417. PMC 5824274. PMID 27930769.
- Kammerer, Christian F.; Masyutin, Vladimir (2018). "A new therocephalian (Gorynychus masyutinae gen. et sp. nov.) from the Permian Kotelnich locality, Kirov Region, Russia". PeerJ. 6: e4933. doi:10.7717/peerj.4933. PMC 5995100. PMID 29900076.
- Wilson, A.; Flint, S.; Payenberg, T.; Tohver, E. (2014). "Architectural Styles and Sedimentology of the Fluvial Lower Beaufort Group, Karoo Basin, South Africa". Journal of Sedimentary Research. 84 (4): 326–348. doi:10.2110/jsr.2014.28.
- Smith, R. M. H. (1987). "Morphology and depositional history of exhumed Permian point bars in the southwestern Karoo, South Africa". Journal of Sedimentary Research. 57 (1): 19–20. doi:10.1306/212F8A8F-2B24-11D7-8648000102C1865D.
- Yakimenko, E.; Inozemtsev, S. A.; Naugolnykh, S. V. (2004). "Upper Permian paleosols (Salarevskian Formation) in the central part of the Russian Platform: Paleoecology and paleoenvironment". Revista Mexicana Ciencias Geologicas. 21 (1): 110–119. (convenience link)
- Benton, M. J. (2018). "Hyperthermal-driven mass extinctions: killing models during the Permian–Triassic mass extinction". Philosophical Transactions of the Royal Society A. 376 (2130). doi:10.1098/rsta.2017.0076. PMC 6127390. PMID 30177561.
- Sookias, R. B.; Butler, R. J.; Benson, R. B. J. (2012). "Rise of dinosaurs reveals major body-size transitions are driven by passive processes of trait evolution". Proceedings of the Royal Society B. 279 (1736): 2180–2187. doi:10.1098/rspb.2011.2441. PMID 22298850. S2CID 1231897.