Frederick Soddy
Frederick Soddy FRS[2] (2 September 1877 – 22 September 1956) was an English radiochemist who explained, with Ernest Rutherford, that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions. He also proved the existence of isotopes of certain radioactive elements.[3][4][5][6][7][8][9][10]
Frederick Soddy | |
|---|---|
 | |
| Born | 2 September 1877 |
| Died | 22 September 1956 (aged 79) Brighton, Sussex, England |
| Nationality | British |
| Alma mater | |
| Known for | |
| Spouse(s) | Winifred Beilby[1] |
| Awards | |
| Scientific career | |
| Fields | |
| Institutions |
|
Biography
Soddy was born at 5 Bolton Road, Eastbourne, England, the son of Benjamin Soddy, corn merchant, and his wife Hannah Green. He went to school at Eastbourne College, before going on to study at University College of Wales at Aberystwyth and at Merton College, Oxford, where he graduated in 1898 with first class honours in chemistry.[1] He was a researcher at Oxford from 1898 to 1900.
Scientific career
In 1900 he became a demonstrator in chemistry at McGill University in Montreal, Quebec, where he worked with Ernest Rutherford on radioactivity.[11][1] He and Rutherford realized that the anomalous behaviour of radioactive elements was because they decayed into other elements. This decay also produced alpha, beta, and gamma radiation. When radioactivity was first discovered, no one was sure what the cause was. It needed careful work by Soddy and Rutherford to prove that atomic transmutation was in fact occurring.
In 1903, with Sir William Ramsay at University College London, Soddy showed that the decay of radium produced helium gas.[1] In the experiment a sample of radium was enclosed in a thin-walled glass envelope sited within an evacuated glass bulb. After leaving the experiment running for a long period of time, a spectral analysis of the contents of the former evacuated space revealed the presence of helium.[12] Later in 1907, Rutherford and Thomas Royds showed that the helium was first formed as positively charged nuclei of helium (He2+) which were identical to alpha particles, which could pass through the thin glass wall but were contained within the surrounding glass envelope.[13]
From 1904 to 1914, Soddy was a lecturer at the University of Glasgow. Ruth Pirret worked as his research assistant during this time.[14] In May 1910 Soddy was elected a Fellow of the Royal Society.[2][15] In 1914 he was appointed to a chair at the University of Aberdeen, where he worked on research related to World War I.
The work that Soddy and his research assistant Ada Hitchins did at Glasgow and Aberdeen showed that uranium decays to radium.[16] It also showed that a radioactive element may have more than one atomic mass though the chemical properties are identical.[17] Soddy named this concept isotope meaning "same place". The word was initially suggested to him by Margaret Todd.[18] Later, J. J. Thomson showed that non-radioactive elements can also have multiple isotopes.
In 1913, Soddy also showed that an atom moves lower in atomic number by two places on alpha emission, higher by one place on beta emission. This was discovered at about the same time by Kazimierz Fajans, and is known as the radioactive displacement law of Fajans and Soddy, a fundamental step toward understanding the relationships among families of radioactive elements. Soddy published The Interpretation of Radium (1909) and Atomic Transmutation (1953).
In 1918 he announced discovery of a stable isotope of Protactinium, working with John Arnold Cranston. This slightly post-dated its discovery by German counterparts; however, it is said their discovery was actually made in 1915 but its announcement was delayed due to Cranston's notes being locked away whilst on active service in the First World War.[19]
In 1919 he moved to the University of Oxford as Dr Lee's Professor of Chemistry, where, in the period up till 1936, he reorganized the laboratories and the syllabus in chemistry. He received the 1921 Nobel Prize in chemistry for his research in radioactive decay and particularly for his formulation of the theory of isotopes.
His work and essays popularising the new understanding of radioactivity was the main inspiration for H. G. Wells's The World Set Free (1914), which features atomic bombs dropped from biplanes in a war set many years in the future. Wells's novel is also known as The Last War and imagines a peaceful world emerging from the chaos. In Wealth, Virtual Wealth and Debt Soddy praises Wells’s The World Set Free. He also says that radioactive processes probably power the stars.
Economics
| Part of a series on |
| Ecological economics |
|---|
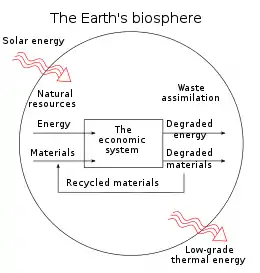 |
In four books written from 1921 to 1934, Soddy carried on a "campaign for a radical restructuring of global monetary relationships",[20] offering a perspective on economics rooted in physics – the laws of thermodynamics, in particular – and was "roundly dismissed as a crank".[20] While most of his proposals – "to abandon the gold standard, let international exchange rates float, use federal surpluses and deficits as macroeconomic policy tools that could counter cyclical trends, and establish bureaus of economic statistics (including a consumer price index) in order to facilitate this effort" – are now conventional practice, his critique of fractional-reserve banking still "remains outside the bounds of conventional wisdom" although a recent paper by the IMF reinvigorated his proposals.[20][21] Soddy wrote that financial debts grew exponentially at compound interest but the real economy was based on exhaustible stocks of fossil fuels. Energy obtained from the fossil fuels could not be used again. This criticism of economic growth is echoed by his intellectual heirs in the now emergent field of ecological economics.[20]
Antisemitic views
In Wealth, Virtual Wealth and Debt Soddy cited the (fraudulent) Protocols of the Learned Elders of Zion as evidence for the belief, which was relatively widespread at the time, of a "financial conspiracy to enslave the world". He used the imagery of a Jewish conspiracy to buttress his claim that "A corrupt monetary system strikes at the very life of the nation." In the same document, he made reference to "the semi-Oriental" who is "supreme" in "high finance" and to an "iridescent bubble of beliefs blown around the world by the Hebraic hierarchy". Later in life he published a pamphlet Abolish Private Money, or Drown in Debt (1939) with a noted publisher of anti-Semitic texts.[22] The influence of his writing can be gauged, for example, in this quote from Ezra Pound:
"Professor Frederick Soddy states that the Gold Standard monetary system has wrecked a scientific age! ... The world's bankers ... have not been content to take their share of modern wealth production – great as it has been – but they have refused to allow the masses of mankind to receive theirs."[23]
Descartes' theorem
He rediscovered the Descartes' theorem in 1936 and published it as a poem, "The Kiss Precise", quoted at Problem of Apollonius. The kissing circles in this problem are sometimes known as Soddy circles.
Honours and awards
He received the Nobel Prize in Chemistry in 1921 and the same year he was elected member of the International Atomic Weights Committee. A small crater on the far side of the Moon as well as the radioactive uranium mineral soddyite are named after him.[24]
Personal life
In 1908, Soddy married Winifred Moller Beilby (1885-1936), the daughter of industrial chemist Sir George Beilby and Lady Emma Bielby, a philanthropist to women's causes. The couple worked together and co-published a paper in 1910 on the absorption of gamma rays from radium.[25] He died in Brighton, England in 1956, twenty days after his 79th birthday.[1]
Bibliography
- Radioactivity (1904)
- The Interpretation of Radium (1909)
- Matter and Energy (1911), second edition (2015)
- The Chemistry of the Radio-elements (1915)
- Science and life: Aberdeen addresses (1920)
- Cartesian Economics: The Bearing of Physical Science upon State Stewardship (1921)
- Science and Life Wealth, Virtual Wealth, and Debt Money versus Man etc (1921)
- Nobel Lecture – The origins of the conception of isotopes (1922)
- Wealth, Virtual Wealth and Debt. The solution of the economic paradox (George Allen & Unwin, 1926)
- The wrecking of a scientific age (1927)
- The Interpretation of the Atom (1932)
- Money versus Man (1933)
- The Role of Money (London: George Routledge & Sons Ltd, 1934) at Internet Archive.org, second edition (2015)
- Money as nothing for something ; The gold "standard" snare (1935)
- Abolish Private Money, or Drown in Debt (1939)
- Present outlook, a warning : debasement of the currency, deflation and unemployment (1944)
- The Story of Atomic Energy (1949)
- Atomic Transmutation (1953)
See also
- Ada Hitchins, who helped Soddy to discover the element protactinium
- Alfred J. Lotka
- Problem of Apollonius
- Oliver Sacks' autobiography Uncle Tungsten, in which Soddy, his work and his profound discoveries in atomic physics are extensively discussed and explained in Sacks' insightful and easily understandable language.
References
- "The Nobel Prize in Chemistry 1921 – Frederick Soddy Biographical". Nobelprize.org. Retrieved 28 November 2017.
- Fleck, A. (1957). "Frederick Soddy Born Eastbourne 2 September 1877 Died Brighton 26 September 1956". Biographical Memoirs of Fellows of the Royal Society. 3: 203–226. doi:10.1098/rsbm.1957.0014. JSTOR 769361.
- Davies, M. (1992). "Frederick Soddy: The scientist as prophet". Annals of Science. 49 (4): 351–367. doi:10.1080/00033799200200301.
- Kauffman, G. B. (1997). "Book Review:The World Made New: Frederick Soddy, Science, Politics, and Environment Linda Merricks". Isis. 88 (3): 564–565. doi:10.1086/383825.
- Daly, H. E. (1980). "The Economic Thought of Frederick Soddy". History of Political Economy. 12 (4): 469–488. doi:10.1215/00182702-12-4-469.
- Freedman, M. I. (2009). "Frederick Soddy and the Practical Significance of Radioactive Matter". The British Journal for the History of Science. 12 (3): 257–260. doi:10.1017/S0007087400017313.
- Sclove, R. E. (1989). "From Alchemy to Atomic War: Frederick Soddy's "Technology Assessment" of Atomic Energy, 1900–1915". Science, Technology & Human Values. 14 (2): 163–194. doi:10.1177/016224398901400203. S2CID 143597812., pp. 163–194
- Linda Merricks (1996). The World Made New: Frederick Soddy, Science, Politics, and Environment. Oxford New York: Oxford University Press. p. 223. ISBN 0-19-855934-8.
- A. N. Krivomazov (1978). Frederick Soddy: 1877–1956. Moscow: Nauka. p. 208.
- George B. Kauffman (1986). Frederick Soddy (1877–1956): Early Pioneer in Radiochemistry (Chemists and Chemistry). Dordrecht; Boston; Hingham: D. Reidel Pub. Co. p. 272. ISBN 978-90-277-1926-3.
- John Gribbin (2014). 13.8: The Quest to Find the True Age of the Universe and the Theory of Everything. London: Icon Books. ISBN 978-1-84831-918-9.
- William Ramsay, Frederick Soddy (1903). "Experiments in Radioactivity, and the Production of Helium from Radium". Proceedings of the Royal Society of London.72.204 – 207
- Pirret, Ruth; Soddy, Frederick (1911). "LXXVII. The ratio between uranium and radium in minerals. II". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 21 (125): 652–658. doi:10.1080/14786440508637078. ISSN 1941-5982.
- "Library and Archive". Royal Society. Retrieved 19 October 2010.
- Soddy, Frederick; Hitchins, A. F. R. (August 1915). "XVII. The relation between uranium and radium.—Part VI. The life-period of ionium". Philosophical Magazine. 6. 30 (176): 209–219. doi:10.1080/14786440808635387.
- Soddy, Frederick (15 February 1917). "The Atomic Weight of "Thorium" Lead". Nature. 98 (2468): 469. Bibcode:1917Natur..98Q.469S. doi:10.1038/098469a0. S2CID 3979761. Retrieved 12 April 2014.
- Britton, Kate (August 2017). "Archaeological Futures: A stable relationship: isotopes and bioarchaeology are in it for the long haul". Antiquity. 91 (358): 853–864. doi:10.15184/aqy.2017.98. hdl:2164/8892. ISSN 0003-598X.
- "University of Glasgow :: Story :: Biography of John Arnold Cranston".
- Zencey, Eric (12 April 2009). "Mr. Soddy's Ecological Economy" (Opinion). The New York Times. Retrieved 22 December 2017.
- Beneš, Jaromír; Kumhof, Michael (August 2012). "The Chicago Plan Revisited". SSRN 2169748. Cite journal requires
|journal=(help) - Frederick Soddy's Economics and the Protocols of the Elders of Zion (1939)
- Surette, Leon (1999). Pound in Purgatory: From Economic Radicalism to Anti-Semitism. University of Illinois Press. p. 218.
- Soddyite Mineral Data
- Ogilvie, Marilyn Bailey; Harvey, Joy Dorothy (2000). The Biographical Dictionary of Women in Science: L-Z. Taylor & Francis. ISBN 978-0-415-92040-7.
External links
| Wikiquote has quotations related to: Frederick Soddy |
- The Central Role of Energy in Soddy's Holistic and Critical Approach to Nuclear Science, Economics, and Social Responsibility
- Annotated bibliography for Frederick Soddy from the Alsos Digital Library for Nuclear Issues
- M. King Hubbert on the Nature of Growth. 1974
- A biography of Frederick Soddy by Arian Forrest Nevin
- The Frederick Soddy Trust
- Frederick Soddy on Nobelprize.org
 including the Nobel Lecture, December 12, 1922 The Origins of the Conception of Isotopes
including the Nobel Lecture, December 12, 1922 The Origins of the Conception of Isotopes - Works by or about Frederick Soddy at Internet Archive
- Works by Frederick Soddy at Faded Page (Canada)
- Frederick Soddy Papers, 1920-1956 (inclusive). H MS c388. Harvard Medical Library, Francis A. Countway Library of Medicine, Boston, Mass.
