Hector's dolphin
Hector's dolphin (Cephalorhynchus hectori) is one of four dolphin species belonging to the genus Cephalorhynchus. Hector's dolphin is the only cetacean endemic to New Zealand, and comprises two subspecies: C. h. hectori, the more numerous subspecies, also referred to as South Island Hector's dolphin; and the critically endangered Māui dolphin (C. h. maui), found off the West Coast of the North Island.[2]
| Hector's dolphin | |
|---|---|
 | |
 | |
| Size compared to an average human | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Artiodactyla |
| Infraorder: | Cetacea |
| Family: | Delphinidae |
| Genus: | Cephalorhynchus |
| Species: | C. hectori |
| Binomial name | |
| Cephalorhynchus hectori Van Beneden, 1881 | |
| Subspecies | |
| |
 | |
Etymology
Hector's dolphin was named after Sir James Hector (1834–1907), who was the curator of the Colonial Museum in Wellington (now the Museum of New Zealand Te Papa Tongarewa). He examined the first specimen of the dolphin found by cephologists. The species was scientifically described by Belgian zoologist Pierre-Joseph van Beneden in 1881. Māori names for Hector's and Māui dolphin include tutumairekurai, tupoupou and popoto.
Description

Hector's dolphin is the smallest dolphin species. Mature adults have a total length of 1.2–1.6 m (3 ft 11 in–5 ft 3 in) and weigh 40–60 kg (88–132 lb).[3] The species is sexually dimorphic, with females being about 5–7% longer than males.[4] The body shape is stocky, with no discernible beak. The most distinctive feature is the rounded dorsal fin, with a convex trailing edge and undercut rear margin.
The overall coloration appearance is pale grey, but closer inspection reveals a complex and elegant combination of colours. The back and sides are predominantly light grey, while the dorsal fin, flippers, and flukes are black. The eyes are surrounded by a black mask, which extends forward to the tip of the rostrum and back to the base of the flipper. A subtly shaded, crescent-shaped black band crosses the head just behind the blowhole. The throat and belly are creamy white, separated by dark-grey bands meeting between the flippers. A white stripe extends from the belly onto each flank below the dorsal fin.
At birth, Hector's dolphin calves have a total length of 60–80 cm (24–31 in) and weigh 8–10 kg (18–22 lb).[5] Their coloration is the almost same as adults, although the grey has a darker hue. Newborn Hector's dolphins have distinct fetal fold marks on their flanks that cause a change in coloration pattern of the skin. These changes are visible for approximately six months and consist of four to six vertical light grey stripes against darker grey skin.[5]
Life history
Data from field studies, beachcast individuals, and dolphins caught in fishing nets have provided information on their life history and reproductive parameters.[3] Photo-ID based observations at Banks Peninsula from 1984 to 2006 show that individuals can reach at least 22 years of age.[6] Males attain sexual maturity between 6 and 9 years old and females begin calving between 7 and 9 years old. Females will continue to calve every 2–3 years, resulting in a maximum of 4–7 calves in one female's lifetime. Calving occurs during the spring and summer.[7] Calves are weaned at around one year of age, and the mortality rate in the first 6 months was estimated to be around 36%.[8]
These combined life-history characteristics mean that, like many other cetaceans, Hector's dolphins are only capable of slow population growth. Their maximum population growth rate was previously estimated to be 1.8–4.9% per year, based on old demographic information,[9] which was then updated to 3–7% per year, based on updated demographic information and a life history invariant observed across all vertebrates [10][11]
Ecology
Habitat
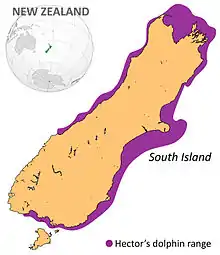
The species' range includes murky coastal waters out to 100 m (330 ft) depth, though almost all sightings are in waters shallower than 50 m (160 ft).[12][13][10] Hector's dolphins display a seasonal inshore-offshore movement; favouring shallow coastal waters during spring and summer, and moving offshore into deeper waters during autumn and winter. They have also been shown to return to the same location during consecutive summers, displaying high foraging site fidelity. The inshore-offshore movement of Hector's dolphins are thought to relate to seasonal patterns of turbidity and the inshore movements of prey species during spring and summer.[14][10]
Diet
Hector's dolphins are generalist feeders, with prey selection based on size (mostly under 10 cm in length) rather than species, although spiny species also appear to be avoided.[15] The largest prey item recovered from a Hector's dolphin stomach was an undigested red cod weighing 500 g with a standard length of 35 cm.[15] The stomach contents of dissected dolphins include a mixture of surface-schooling fish, midwater fish, squid, and a variety of benthic species.[15] The main prey species in terms of mass contribution is red cod, and other important prey include Peltorhamphus flatfish, ahuru, New Zealand sprat, Nototodarus arrow squid, and juvenile giant stargazer.[15][14][16]
Predators
The remains of Hector's dolphins have been found in the stomachs of broadnose sevengill shark (considered to be their main predator),[10] great white shark and blue shark.[17][18] Unconfirmed predators of Hector's and Māui dolphins include killer whales (orca), mako sharks and bronze whaler shark.[19]
Behaviour
Group dynamics
Hector's dolphins preferentially form groups of less than 5 individuals, with a mean of 3.8 individuals, that are highly segregated by sex. The majority of these small groups are single sex. Groups of greater than 5 individuals are formed much less frequently. These larger groups, >5, are usually mixed sex and have been shown to form only to forage or participate in sexual behavior. Nursery groups can also be observed and are usually all female groups of less than 7 mother and young.[4]
This species has been found to be show a high level of fluidity with weak inter-individual associations, meaning they do not form strong bonds with other individuals. Three types of small preferential groups have been found: nursery groups; immature and subadult groups; and adult male/female groups. All of these small groups show a high level of sex segregation. Hector's dolphins display a sex-age population group composition, meaning they group by biological sex and age.[8]

Sexual behaviour
Males of the species have extremely large testes in proportion to body size, with the highest relative weight in one study being 2.9% of body weight. Large testes in combination with males' smaller overall body size suggests a promiscuous mating system. This type of reproductive system would involve a male attempting to fertilize as many females as possible and little male-male aggression. The amount of sexual behavior per individual in the species is observed most when small single sex groups form large mixed sex groups. Sexual behavior in the species is usually non-aggressive.[7]
Echolocation
Similar to the hourglass dolphin, Hector's dolphins use high-frequency echolocation clicks. However, the Hector's dolphin produces lower source-level clicks than hourglass dolphins due to their crowded environment. This means they can only spot prey at half the distance compared to an hourglass dolphin.[20] The species has a very simple repertoire with few types of clicks, as well as little audible signals in addition to these. More complex clicks could be observed in large groups.[21]
Distribution and population size
Hector's and Māui dolphins are endemic to the coastal regions of New Zealand. The Hector's dolphin sub-species is most abundant in discontinuous regions of high turbidity around the South Island. They are most abundant off the East Coast and West Coast, most notably around Banks Peninsula, with smaller, more isolated populations off the North Coast and South Coast (notably at Te Waewae Bay).[22][23] Smaller populations are scattered around the South Island, including: Cook Strait, Kaikoura, Catlins (e.g., Porpoise Bay, Curio Bay), and Otago coasts (e.g.Karitane, Oamaru, Moeraki, Otago Harbour, and Blueskin Bay).[24] Māui dolphin are typically found on the west coast of the North Island between Maunganui Bluff and Whanganui.[25]
An aerial survey of South Island Hector's dolphin abundance—which was commissioned by the Ministry for Primary Industries, carried out the Cawthron Institute, and endorsed by the International Whaling Commission—estimated a total population size of 14,849 dolphins (95% confidence interval = 11,923–18,492).[26] This was almost twice the previous, published estimate from earlier surveys (7,300; 95% CI 5,303–9,966). This difference was primarily due to a much larger estimated population along East Coast, which was distributed further offshore than previously thought.[27]
The latest estimate of the Māui dolphin subspecies is 63 individuals of age 1 year or older (95% CL = 57, 75).[28]
Mixing of sub-species
Occasionally, South Island Hector's dolphins (determined from genetics) are found around the North Island, up to Bay of Plenty or Hawke's Bay.[29] In 2012, a genetic analysis of tissue samples from dolphins in the core Maui dolphin range, including historical samples, revealed the presence of at least three South Island Hector's dolphins off the West Coast of the North Island (two of them alive), along with another five South Island Hector's dolphins sampled between Wellington and Oakura from 1967 to 2012.[30]
Previously, the deep waters of the Cook Strait were considered to be an effective barrier to mixing between the South Island Hector's and North Island Māui sub-species for around 15,000 and 16,000 years. This is coincident with the separation of the North and South Islands of New Zealand at the end of the last ice age.[31] To date, there is no evidence of interbreeding between South Island Hector's dolphin and Māui dolphin,[30][32] but it is likely they could given their close genetic composition.
Threats
Fishing
Hector's and Māui dolphin deaths occur as a direct result of commercial and recreational fishing due to entanglement or capture in gillnets or trawls.[33] Death is ultimately caused by suffocation, although injury and sub-lethal effects can also result from the mechanical abrasion of fins resulting from entanglement. Since the 1970s, gillnets have been made from lightweight monofilament, which is difficult for dolphins to detect. Hector's dolphins are actively attracted to trawling vessels and can frequently be seen following trawlers and diving down to the net, which could result in the unwanted bycatch.[34]
Deaths in fishing nets were previously considered to be the most serious threat (responsible for more than 95% of the human-caused deaths in Māui dolphins), with currently lower level threats including tourism, disease, and marine mining.[35][36] Research of decreases in mitochondrial DNA diversity among hector's dolphin populations has suggested that the amount of gill-net entanglement deaths likely far surpasses that reported by fisheries.[37] Population simulations estimated that the current population is 30% of the 1970 population size estimate of 50,000 dolphins, based on their estimated capture rate in commercial gillnet fisheries.[38]
The latest government-approved estimates of annual deaths in commercial gillnets (for the period from 2014/15 to 2016/17) was 19–93 South Island Hector's dolphins and 0.0–0.3 Māui dolphins annually.[10] The low estimate for Māui dolphin deaths in gillnets is consistent with the lack of any observed captures in commercial setnets off the West Coast of the North Island since late-2012, despite 100% observer coverage in this fishery across this time period. Annual deaths in commercial trawls were estimated to be 0.2–26.6 Hector's dolphins and 0.00–0.05 Maui dolphins (from 2014/15 to 2016/17). Based on these levels of mortality, the increased abundance of Hector's dolphins and faster population growth potential than previously thought, the commercial fishery threat (alone) would be unlikely to prevent population recovery to at least 80% of unimpacted levels, for either Hector's or Māui dolphins.[10] However the threat from commercial fishing was estimated to be higher for some regional populations relative to others, e.g., East Coast South Island, and may have a greater effect on certain smaller populations, e.g., Hector's dolphins along the Kaikoura Coast.[10]
Fishing restrictions
The first marine protected area (MPA) for Hector's dolphin was designated in 1988 at Banks Peninsula, where commercial gill-netting was effectively prohibited out to 4 nmi (7.4 km; 4.6 mi) offshore and recreational gill-netting was subject to seasonal restrictions. A second MPA was designated on the west coast of the North Island in 2003. Populations continued to decline due to by-catch outside the MPAs.[25]
Additional protection was introduced in 2008, banning gill-netting within 4 nautical miles of the majority of the South Island's east and south coasts, out to 2 nautical miles (3.7 km) offshore off the South Island's west coast and extending the gillnet ban on the North Island's west coast to 7 nmi (13 km; 8.1 mi) offshore. Also, restrictions were placed on trawling in some of these areas. For further details on these regulations, see the Ministry of Fisheries website.[39] Five marine mammal sanctuaries were designated in 2008 to manage nonfishing-related threats to Hector's and Māui dolphins.[40] Their regulations include restrictions on mining and seismic acoustic surveys. Further restrictions were introduced into Taranaki waters in 2012 and 2013 to protect Māui dolphins.[41]
The Scientific Committee of the International Whaling Commission has recommended extending protection for Māui dolphin further south to Whanganui and further offshore to 20 nautical miles from the coastline. The IUCN has recommended protecting Hector's and Māui dolphins from gill-net and trawl fisheries, from the shoreline to the 100 m depth contour.
Infectious diseases
The unicellular parasiteToxoplasma gondii is considered to be the main non-fishery cause of death. A 2013 study found that seven of 28 beachcast or bycaught Hector's and Māui dolphins dolphins died as a result of toxoplasmosis, which had necrotising and haemorrhagic lesions in the lung (n = 7), lymph nodes (n = 6), liver (n = 4) and adrenals (n = 3).[42] The same study found that approximately two-thirds of dolphins had previously been infected with the toxoplasma parasite. An update to this study found that toxoplasmosis had killed nine out of 38 post-weaning age Hector's and Māui dolphins found washed up or floating at-sea, and that were not too autolised to determine a cause of death.[43] Of these nine, six were reproductive females, tentatively indicating that this demographic may be more susceptible to infection.[10] In New Zealand, the domestic house cat is the only known definitive host for toxoplasma, and Hector's and Maui dolphins are thought to become infected as a result of their preference for turbid coastal waters near river mouths, where toxoplasma oocyst densities are likely to be relatively high.[10]
Brucellosis is a notable bacterial disease of Hector's and Māui dolphins that can cause late pregnancy abortion in terrestrial mammals, and has been found in a range of cetacean species elsewhere.[44] Brucellosis has been determined from necropsies to have killed both Hector's and Māui dolphins and to have caused reproductive disease, indicating that it may affect the reproductive success of both sub-species.[45][10]
Loss of genetic diversity and population decline
The high levels of sex segregation and fragmentation of different populations in Hector's dolphin have been discussed as contributing to the overall population decline, as it becomes more difficult for males to find a female and copulate. The Allee effect begins to occur when a low-density population has low reproductive rates leading to increased population decline.[4] In addition, low gene flow between populations may result from this species' high foraging site fidelity. Hector's dolphins have not been found to participate in alongshore migrations, which may also contribute to their lack of genetic diversity.
Samples from 1870 to today have provided a historical timeline for the species' population decline. Lack of neighboring populations due to fishery-related mortality has decreased gene flow and contributed to an overall loss in mitochondrial DNA diversity. As a result, the populations have become fragmented and isolated, leading to inbreeding. The geographical range has been lessened to the point where gene flow and immigration may no longer be possible between Māui dolphin and Hector's dolphin.[37][46]
Potential interbreeding between Hector's and Māui dolphins could increase the numbers of dolphins in the Māui range and reduce the risk of inbreeding depression, but such interbreeding could eventually result in a hybridisation of the Māui back into the Hector's species and lead to a reclassification of Māui as again the North Island Hector's. Hybridisation in this manner threatens the Otago black stilt[47] and the Chatham Islands' Forbes parakeet[48] and has eliminated the South Island brown teal as a subspecies.[49] Researchers have also identified potential interbreeding as threatening the Māui with hybrid breakdown and outbreeding depression.
References
- Reeves, R.R.; Dawson, S.M.; Jefferson, T.A.; Karczmarski, L.; Laidre, K.; O'Corry-Crowe, G.; Rojas-Bracho, L.; Secchi, E.R.; Slooten, E.; Smith, B.D.; Wang, J.Y. & Zhou, K. (2013). "Cephalorhynchus hectori". IUCN Red List of Threatened Species. 2013: e.T4162A44199757. doi:10.2305/IUCN.UK.2013-1.RLTS.T4162A44199757.en.
- Baker, Alan N.; Smith, Adam N.H.; Pichler, Franz B. (2002). "Geographical variation in Hector's dolphin: recognition of a new subspecies of Cephalorhynchus hectori". Journal of the Royal Society of New Zealand. 32 (4): 713–727. CiteSeerX 10.1.1.113.9489. doi:10.1080/03014223.2002.9517717.
- Slooten, E. and Dawson, S.M. 1994. Hector's dolphin Cephalorhynchus hectori. Pp. 311–333 in: Handbook of Marine Mammals. Volume V (Delphinidae and Phocoenidae) (S.H. Ridgway and R. Harrison eds). Academic Press. New York.
- Webster, T.A.; Dawson, S.M.; Slooten, E. (2009). Evidence of Sex Segregation in Hector’s Dolphin (Cehalorhynchus hectori). Aquatic Mammals. Vol. 35, Iss. 2.: 212–219.
- Slooten, E. 1991. Age, growth and reproduction in Hector's dolphins. Canadian Journal of Zoology 69: 1689–1700.
- Gormley, A (2009). Population Modelling of Hector's Dolphins (Ph.D. thesis). University of Otago.
- Slooten, E. (1991). Age, growth, and reproduction in Hector's dolphins. Can J. Zool. 69(6): 1689–1700.
- Bräger, S. H.-J. (1998). Behavioural ecology and population structure of Hector’s dolphin (Cephalorhynchus hectori) (Thesis, Doctor of Philosophy). University of Otago.
- Slooten, E (1991). "Population biology and conservation of Hector's dolphins". Canadian Journal of Zoology. 69: 1701–1707.
- Roberts, J (2019). Spatial risk assessment of threats to Hector’s and Māui dolphins (Cephalorhynchus hectori) (Report). New Zealand Ministry for Primary Industries and Department of Conservation.
- Dillingham, P (2016). "Improved estimation of intrinsic growth rmax for long‐lived species: integrating matrix models and allometry". Ecological Applications. 26: 322–333.
- Bräger, S., Harraway, J. and Manly, B.F.J. 2003. Habitat selection in a coastal dolphin species (Cephalorhynchus hectori). Marine Biology 143: 233–244.
- Rayment, W., Dawson, S. and Slooten, E. In press. Seasonal changes in distribution of Hector's dolphins at Banks Peninsula, New Zealand: implications for protected area design. Aquatic Conservation: Marine and Freshwater Ecosystems. doi:10.1002/aqc.1049.
- Miller, E (2015). "Ecology of Hector's dolphin (Cephalorhynchus hectori): Quantifying diet and investigating habitat selection at Banks Peninsula". Retrieved 20 August 2019.
- Miller, Elanor; Lalas, Chris; Dawson, Steve; Ratz, Hiltrun; Slooten, Elisabeth (August 2012). "Hector's dolphin diet: The species, sizes and relative importance of prey eaten by Cephalorhynchus hectori, investigated using stomach content analysis". Marine Mammal Science. 29 (4): 606–628. doi:10.1111/j.1748-7692.2012.00594.x – via Researchgate.
- Miller, E (2013). "Hector's dolphin diet: The species, sizes and relative importance of prey eaten by Cephalorhynchus hectori, investigated using stomach content analysis" (PDF). Retrieved 20 August 2019.
- Cawthorn, M (1988). Recent observations of Hector's dolphin Cephalorhynchus hectori, in New Zealand (Report). International Whaling Commission.
- Banks Peninsula Marine Mammal Sanctuary Technical Report. Department of Conservation. 1992. pp. B–9. ISBN 978-0-478-01404-4.
- "Natural threats". www.doc.govt.nz. 2021. Retrieved 31 January 2021.
- Kyhn, L.A.; Tougaard, J.; Jensen, F.; Wahlberg, M.; Stone, G.; Yoshinaga, A.; Beedholm, K.; Madsen, P.T. 2009: Feeding at a high pitch: source parameters of narrow band, high-frequency clicks from echolocating off-shore hourglass dolphins and coastal Hector's dolphins. Journal of the Acoustical Society of America 125(3): 1783–1791.
- Dawson, S.M. (1991) Clicks and Communication: The Behavioural and Social Contexts of Hector's Dolphin Vocalizations. Ethology. Vol 88, Iss. 4.
- Slooten, E., Dawson, S.M. and Rayment, W.J. 2004. Aerial surveys for coastal dolphins: abundance of Hector’s dolphins off the South Island West Coast, New Zealand. Marine Mammal Science 20:477–490.
- Dawson, S.M., Slooten, E., DuFresne, S.D., Wade, P. and Clement, D.M. 2004. Small-boat surveys for coastal dolphins: Line-transect surveys of Hector’s dolphins (Cephalorhynchus hectori). Fishery Bulletin 102: 441–451.
- Slooten L.. Benjamins S.. Turek J.. 2011. Potential impacts of Project Next Generation on Hector’s'dolphins and other marine mammals. Otago University. Retrieved 4 November 2014
- Slooten, E., Dawson, S.M., Rayment, W. and Childerhouse, S. 2006. "A new abundance estimate for Maui's dolphin: What does it mean for managing this critically endangered species?". Biological Conservation 128: 576–581.
- Report of the Scientific Committee, 2017. Journal of Cetacean Research and Management 18
- "Boost in numbers of Hector's dolphinsStuff". Retrieved 10 August 2016.
- Baker, C.S.; Steel, D.; Hamner, R.M.; Hickman, G.; Boren, L.; Arlidge, W.; Constantine, R "Estimating the abundance and effective population size of Māui dolphins using microsatellite genotypes in 2015–16, with retrospective matching to 2001–16 (2016)" (PDF). Retrieved 8 June 2019.
- Tait M.. 2012. Creatures lurking in Bay waters. the Hawkes Bay Today. Retrieved 4 November 2014
- Hamner, Rebecca M.; Oremus, Marc; Stanley, Martin; Brown, Phillip; Constantine, Rochelle; Baker, C. Scott (March 2012). "Estimating the abundance and effective population size of Maui's dolphins using microsatellite genotypes in 2010–11, with retrospective matching to 2001–07" (PDF). www.doc.govt.nz. New Zealand Department of Conservation. Retrieved 31 January 2021.
- Hamner, Rebecca M.; Pichler, Franz B.; Heimeier, Dorothea; Constantine, Rochelle; Baker, C. Scott (August 2012). "Genetic differentiation and limited gene flow among fragmented populations of New Zealand endemic Hector's and Maui's dolphins". Conservation Genetics. 13 (4): 987–1002. doi:10.1007/s10592-012-0347-9.
- Hamner, Rebecca M.; Constantine, Rochelle; Oremus, Marc; Stanley, Martin; Brown, Phillip; Baker, C. Scott (2013). "Long-range movement by Hector's dolphins provides potential genetic enhancement for critically endangered Maui's dolphin". Marine Mammal Science. 30: 139–153. doi:10.1111/mms.12026.
- Starr, P. and Langley, A. 2000. Inshore Fishery Observer Programme for Hector's dolphins in Pegasus Bay, Canterbury Bight, 1997/1998. Published client report on contract 3020, funded by Conservation Services Levy. Department of Conservation, Wellington. 28p.
- Rayment, William, and Trudi Webster. "Observations of Hector's Dolphins () Associating with Inshore Fishing Trawlers at Banks Peninsula, New Zealand." New Zealand Journal of Marine and Freshwater Research 43.4 (2009): 911–16. Web.
- Bejder, L., Dawson, S.M. and Harraway, J.A. 1999. Responses by Hector's dolphins to boats and swimmers in Porpoise Bay, New Zealand. Marine Mammal Science 15: 738–750.
- Stone, G. S. and Yoshinaga, A. 2000. Hector's dolphin (Cephalorhynchus hectori) calf mortalities may indicate new risks from boat traffic and habituation. Pacific Conservation Biology 6: 162–170.
- Pichler, F.B.; Baker, C.S. (2000). Loss of genetic diversity in the endemic Hector’s dolphin due to fisheries-related mortality. School of Biological Sciences, University of Auckland.
- Slooten, E. and Dawson, S.M.: Updated population viability analysis, population trends and PBRs for Hector's and Maui dolphin. https://www.regulations.gov/document?D=NOAA-NMFS-2016-0118-0076
- "Hector's Dolphins". Ministry of Fisheries. 1 October 2008. Retrieved 16 February 2010.
- "Marine mammal sanctuaries: Marine protected areas". Department of Conservation. Retrieved 16 February 2010.
- Smith, Nick; Guy, Nathan. "Additional protections and survey results good news for dolphins". beehive.govt.nz. New Zealand Government.
- Roe, W.D., L. Howe, E.J. Baker, L. Burrows, and S.A. Hunter. "An Atypical Genotype of Toxoplasma Gondii as a Cause of Mortality in Hector's Dolphins (Cephalorhynchus Hectori)." Veterinary Parasitology 192.1–3 (2013): 67–74. Web.
- "Scientists reach new understanding of main threats to Hector's and Māui dolphins". Retrieved 7 June 2019.
- "Natural threats". New Zealand Department of Conservation. Retrieved 4 May 2017.
- Buckle, K (2017). "Brucellosis in Endangered Hector's Dolphins (Cephalorhynchus hectori)". Veterinary Pathology. 54 (5): 838–845. doi:10.1177/0300985817707023. PMID 28494705.
- Pichler, F.B.; Dawson, S.M.; Slooten, E.; Baker, C.S. (2008). Geographic Isolation of Hector’s Dolphin Populations Described by Mitochondrial DNA Sequences. Conservation Biology. Vol 2, Iss. 3.
- Wallis, G. "Genetic status of New Zealand black stilt (Himantopus novaezelandiae ) and impact of hybridisation" (PDF). New Zealand Department of Conservation.
- Greene, T. C. (2000). "Forbes' parakeet (Cyanoramphus forbesi) population on Mangere Island, Chatham Islands" (PDF). www.doc.govt.nz/. New Zealand Department of Conservation. Retrieved 31 January 2021.
- Gemmel, Neil J.; Flint, Heather J. (2000). "Taxonomic status of the brown teal (Anas chlorotis) in Fiordland" (PDF). www.doc.govt.nz. New Zealand Department of Conservation. Retrieved 31 January 2021.
Further reading
- National Audubon Society: Guide to Marine Mammals of the World ISBN 0-375-41141-0
- Encyclopedia of Marine Mammals ISBN 0-12-551340-2
- Whales, Dolphins and Porpoises, Mark Carwardine 1995 ISBN 0-7513-2781-6
- Facts about Hector's dolphins Department of Conservation – Several Images & listed as 'critically endangered' – Retrieved 8 May 2007.
- Hector's Dolphins, New Zealand Ministry of Fisheries – Retrieved 9 February 2007.
- Hector's Dolphin – Factsheet, Royal Forest and Bird Protection Society of New Zealand Inc. – Retrieved 9 February 2007.
External links
| Wikimedia Commons has media related to Cephalorhynchus hectori. |
- Specimen MNZ MM001915, collected Kaikoura, New Zealand, no date data
- NZ Dept. of Conservation – Hector's dolphin information
- NABU International www.hectorsdolphins.com
- Three decades on the tail of Hector's dolphins
