Even-toed ungulate
The even-toed ungulates (Artiodactyla /ˌɑːrtioʊˈdæktɪlə/, from Ancient Greek ἄρτιος, ártios 'even', and δάκτυλος, dáktylos 'finger / toe') are ungulates—hoofed animals—which bear weight equally on two (an even number) of their five toes: the third and fourth. The other three toes are either present, absent, vestigial, or pointing posteriorly. By contrast, odd-toed ungulates bear weight on one (an odd number) of the five toes: the third toe. Another difference between the two is that even-toed ungulates digest plant cellulose in one or more stomach chambers rather than in their intestine as the odd-toed ungulates do.
| Even-toed ungulates | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Magnorder: | Boreoeutheria |
| Clade: | Laurasiatheria |
| Clade: | Scrotifera |
| Clade: | Ferungulata |
| Clade: | Ungulata |
| Order: | Artiodactyla Owen, 1848 |
| Subdivisions | |
The aquatic cetaceans (whales, dolphins, and porpoises) evolved from even-toed ungulates, so modern taxonomic classification combines the two under the name Cetartiodactyla /sɪˌtɑːrtioʊˈdæktɪlə/.
The roughly 270 land-based even-toed ungulate species include pigs, peccaries, hippopotamuses, antelopes, mouse deer, deer, giraffes, camels, llamas, alpacas, sheep, goats, and cattle. Many of these are of great dietary, economic, and cultural importance to humans.
Evolution
The oldest fossils of even-toed ungulates date back to the early Eocene (about 53 million years ago). Since these findings almost simultaneously appeared in Europe, Asia, and North America, it is very difficult to accurately determine the origin of artiodactyls. The fossils are classified as belonging to the family Dichobunidae; their best-known and best-preserved member is Diacodexis.[1] These were small animals, some as small as a hare, with a slim build, lanky legs, and a long tail. Their hind legs were much longer than their front legs. The early to middle Eocene saw the emergence of the ancestors of most of today's mammals.[2]

Two formerly widespread, but now extinct, families of even-toed ungulates were Entelodontidae and Anthracotheriidae. Entelodonts existed from the middle Eocene to the early Miocene in Eurasia and North America. They had a stocky body with short legs and a massive head, which was characterized by two humps on the lower jaw bone. Anthracotheres had a large, porcine (pig-like) build, with short legs and an elongated muzzle. This group appeared in the middle Eocene up until the Pliocene, and spread throughout Eurasia, Africa, and North America. Anthracotheres are thought to be the ancestors of hippos, and, likewise, probably led a similar aquatic lifestyle. Hippopotamuses appeared in the late Miocene and occupied Africa and Asia—they never got to the Americas.[2]
The camels (Tylopoda) were, during large parts of the Cenozoic, limited to North America; early forms like Cainotheriidae occupied Europe. Among the North American camels were groups like the stocky, short-legged Merycoidodontidae. They first appeared in the late Eocene and developed a great diversity of species in North America. Only in the late Miocene or early Pliocene did they migrate from North America into Eurasia. The North American varieties became extinct around 10,000 years ago.
Suina (including pigs) have been around since the Eocene. In the late Eocene or the Oligocene, two families stayed in Eurasia and Africa; the peccaries, which became extinct in the Old World, exist today only in the Americas.

South America was settled by even-toed ungulates only in the Pliocene, after the land bridge at the Isthmus of Panama formed some three million years ago. With only the peccaries, lamoids (or llamas), and various species of capreoline deer, South America has comparatively fewer artiodactyl families than other continents, except Australia, which has no native species.
Taxonomy and phylogeny

The classification of artiodactyls was hotly debated because the ocean-dwelling cetaceans evolved from the land-dwelling even-toed ungulates. Some semiaquatic even-toed ungulates (hippopotamuses) are more closely related to the ocean-dwelling cetaceans than to the other even-toed ungulates.
This makes the Artiodactyla as traditionally defined a paraphyletic taxon, since it includes animals descended from a common ancestor, but does not include all of its descendants. Phylogenetic classification only recognizes monophyletic taxa; that is, groups that descend from a common ancestor and include all of its descendants. To address this problem, the traditional order Artiodactyla and infraorder Cetacea are sometimes subsumed into the more inclusive Cetartiodactyla taxon.[3] An alternative approach is to include both land-dwelling even-toed ungulates and ocean-dwelling cetaceans in a revised Artiodactyla taxon.[2]
Classification
- Order Artiodactyla/Clade Cetartiodactyla[2][4]
- Suborder Tylopoda
- Family †Anoplotheriidae?
- Family †Cainotheriidae
- Family †Merycoidodontidae
- Family †Agriochoeridae
- Family Camelidae: camels and lamoids or llamas (7 extant and 13 extinct species)
- Family †Oromerycidae
- Family †Xiphodontidae
- Clade Artiofabula
- Suborder Suina
- Family Suidae: pigs (19 species)
- Family Tayassuidae: peccaries (4 species)
- Family †Sanitheriidae
- Clade Cetruminantia
- Clade Cetancodontamorpha
- Genus †Andrewsarchus?
- Family †Entelodontidae
- Suborder Whippomorpha
- Family †Raoellidae
- Superfamily Dichobunoidea – paraphyletic to Cetacea and Raoellidae
- Family †Dichobunidae
- Family †Helohyidae
- Family †Choeropotamidae
- Family †Cebochoeridae
- Family †Mixtotheriidae
- Infraorder Ancodonta
- Family †Anthracotheriidae – paraphyletic to Hippopotamidae
- Family Hippopotamidae: hippos (two species)
- Infraorder Cetacea: whales (about 90 species)
- Parvorder †Archaeoceti
- Family †Pakicetidae
- Family †Ambulocetidae
- Family †Remingtonocetidae
- Family †Basilosauridae
- Parvorder Mysticeti: baleen whales
- Superfamily Balaenoidea: right whales
- Family Balaenidae: greater right whales (four species)
- Family Cetotheriidae: pygmy right whale (one species)
- Superfamily Balaenopteroidea: large baleen whales
- Family Balaenopteridae: slender-back rorquals and humpback whale (eight species)
- Family Eschrichtiidae: gray whale (one species)
- Superfamily Balaenoidea: right whales
- Parvorder Odontoceti: toothed whales
- Superfamily Delphinoidea: oceanic dolphins, porpoises, and others
- Family Delphinidae: oceanic true dolphins (38 species)
- Family Monodontidae: Arctic whales; narwhal and beluga (two species)
- Family Phocoenidae: porpoises (six species)
- Superfamily Physeteroidea: sperm whales
- Family Kogiidae: lesser sperm whales (two species)
- Family Physeteridae: sperm whale (one species)
- Superfamily Platanistoidea: river dolphins
- Family Iniidae: South American river dolphins (two species)
- Family Lipotidae: Chinese river dolphin (one species, possibly extinct)
- Family Platanistidae: South Asian river dolphin (one species)
- Family Pontoporiidae: La Plata dolphin (one species)
- Superfamily Ziphioidea
- Family Ziphidae: beaked whales (22 species)
- Superfamily Delphinoidea: oceanic dolphins, porpoises, and others
- Parvorder †Archaeoceti
- Clade Ruminantiamorpha
- Suborder Ruminantia
- Infraorder Tragulina
- Family †Amphimerycidae
- Family †Prodremotheriidae
- Family †Protoceratidae
- Family †Hypertragulidae
- Family †Praetragulidae
- Family Tragulidae: chevrotains (ten species)
- Family †Archaeomerycidae
- Family †Lophiomerycidae
- Infraorder Pecora
- Family †Gelocidae
- Family †Palaeomerycidae
- Family Antilocapridae: pronghorn (one species)
- Family †Climacoceratidae
- Family Giraffidae: okapi and four species of giraffe (five species total)
- Family †Hoplitomerycidae
- Family Cervidae: deer (49 species)
- Family †Leptomerycidae
- Family Moschidae: musk deer (seven species)
- Family Bovidae: cattle, buffalo, goat-antelope, antelope, and others (135 species)
- Infraorder Tragulina
- Suborder Ruminantia
- Clade Cetancodontamorpha
- Suborder Suina
- Suborder Tylopoda
Research history

In the 1990s, biological systematics used not only morphology and fossils to classify organisms, but also molecular biology. Molecular biology involves sequencing an organism's DNA and RNA and comparing the sequence with that of other living beings—the more similar they are, the more closely they are related. Comparison of even-toed ungulate and cetaceans genetic material has shown that the closest living relatives of whales and hippopotamuses is the paraphyletic group Artiodactyla.
Dan Graur and Desmond Higgins were among the first to come to this conclusion, and included a paper published in 1994.[5] However, they did not recognize hippopotamuses and classified the ruminants as the sister group of cetaceans. Subsequent studies established the close relationship between hippopotamuses and cetaceans; these studies were based on casein genes,[6] SINEs,[7] fibrinogen sequences,[8] cytochrome and rRNA sequences,[3][9] IRBP (and vWF) gene sequences,[10] adrenergic receptors,[11] and apolipoproteins.[12]
In 2001, the fossil limbs of a Pakicetus (amphibioid cetacean the size of a wolf) and Ichthyolestes (an early whale the size of a fox) were found in Pakistan. They were both archaeocetes ("ancient whales") from about 48 million years ago (in the Eocene). These findings showed that archaeocetes were more terrestrial than previously thought, and that the special construction of the talus (ankle bone) with a double-rolled joint surface, previously thought to be unique to even-toed ungulates, were also in early cetaceans.[13] The mesonychids, another type of ungulate, did not show this special construction of the talus, and thus was concluded to not have the same ancestors as cetaceans.

The oldest cetaceans date back to the early Eocene (53 million years ago), whereas the oldest known hippopotamus dates back only to the Miocene (15 million years ago). Some doubts have arisen regarding the relationship between the two, as there is a 40-million-year gap between their first appearances in the fossil record. It seems unlikely that there were ancestral hippos that left no remains, given the high number of even-toed ungulate fossils. Some studies proposed the late emergence of hippos is because they are relatives of peccaries and split recently, but molecular findings contradict this. Research is therefore focused on anthracortheres (family Anthracotheriidae); one dating from the Eocene to Miocene was declared to be "hippo-like" upon discovery in the 19th century. A study from 2005 showed that the anthracotheres and hippopotamuses have very similar skulls, but differed in the adaptations of their teeth. It was nevertheless believed that cetaceans and anthracothereres descended from a common ancestor, and that hippopotamuses developed from anthracotheres. A study published in 2015 was able to confirm this, but also revealed that hippopotamuses were derived from older anthracotheriens.[9][14] The newly introduced genus Epirigenys from eastern Africa is thus the sister group of hippos.
Morphological classification of Artiodactyla
Linnaeus postulated a close relationship between camels and ruminants as early as the mid-1700s. Henri de Blainville recognized the similar anatomy of the limbs of pigs and hippos, and British zoologist Richard Owen coined the term "even-toed ungulates" and the scientific name "Artiodactyla" in 1848.
Internal morphology (mainly the stomach and the molars) were used for classification. Suines (including pigs) and hippopotamuses have molars with well-developed roots and a simple stomach that digests food. Thus, they were grouped together as non-ruminants (Porcine). All other even-toed ungulates have molars with a selenodont construction (crescent-shaped cusps) and have the ability to ruminate, which requires regurgitating food and re-chewing it. Differences in stomach construction indicated that rumination evolved independently between tylopods and ruminants; therefore, tylopods were excluded from Ruminantia.
The taxonomy that was widely accepted by the end of the 20th century was:[15]
Even-toed ungulates |
| ||||||||||||||||||||||||
Morphological classification of Cetacea

Modern cetaceans are highly adapted sea creatures which, morphologically, have little in common with land mammals; they are similar to other marine mammals, such as seals and sea cows, due to convergent evolution. However, they evolved from originally terrestrial mammals. The most likely ancestors were long thought to be mesonychids—large, carnivorous animals from the early Cenozoic (Paleocene and Eocene), which had hooves instead of claws on their feet. Their molars were adapted to a carnivorous diet, resembling the teeth in modern toothed whales, and, unlike other mammals, have a uniform construction.
The suspected relations can be shown as follows:[14][16]
| Paraxonia |
| ||||||||||||
Inner systematics
Molecular findings and morphological indications suggest that artiodactyls as traditionally defined are paraphyletic with respect to cetaceans. Cetaceans are deeply nested within the former; the two groups together form a monophyletic taxon, for which the name Cetartiodactyla is sometimes used. Modern nomenclature divides Artiodactyla (or Cetartiodactyla) in four subordinate taxa: camelids (Tylopoda), pigs and peccaries (Suina), ruminants (Ruminantia), and hippos plus whales (Whippomorpha).
The presumed lineages within Artiodactyla can be represented in the following cladogram:[17][18][19][20][21]
| Artiodactyla |
| ||||||||||||||||||||||||||||||


The four summarized Artiodactyla taxa are divided into ten extant families:[22]
- The camelids (Tylopoda) comprise only one family, Camelidae. It is a species-poor artiodactyl suborder of North American origin[23] that is well adapted to extreme habitats—the dromedary and Bactrian camels in the Old World deserts and the guanacos, llamas, vicuñas, and alpacas in South American high mountain regions.
- The pig-like creatures (Suina) are made up of two families:
- The pigs (Suidae) are limited to the Old World. These include the wild boar and the domesticated form, the domestic pig.
- The peccaries (Tayassuidae) are named after glands on their belly and are indigenous to Central and South America.
- The ruminants (Ruminantia) consist of six families:
- The mouse deer (Tragulidae) are the smallest and most primitive even-toed-ruminants; they inhabit forests of Africa and Asia.
- The giraffe-like creatures (Giraffidae) are composed of two species: the giraffe and the okapi.
- The musk deer (Moschidae) is indigenous to East Asia.
- The antilocaprids (Antilocapridae) of North America comprise only one extant species: the pronghorn.
- The deer (Cervidae) are made up of about 45 species, which are characterized by a pair of antlers (generally only in males). They are spread across Europe, Asia, and the Americas. This group includes, among other species, the red deer, moose, elk (wapiti), and reindeer (caribou).
- The bovids (Bovidae) are the most species-rich. Among them are cattle, sheep, caprines, and antelopes.
- The whippomorphans include hippos and cetaceans:
- The hippos (Hippopotamidae) comprise two groups, the hippo and the pygmy hippo.
- The whales (Cetacea) comprise 72 species and two parvorders: toothed whales (Odontoceti) and baleen whales (Mysticeti)
Although deer, musk deer, and pronghorns have traditionally been summarized as cervids (Cervioidea), molecular studies provide different—and inconsistent—results, so the question of phylogenetic systematics of infraorder Pecora (the horned ruminants) for the time being, cannot be answered.
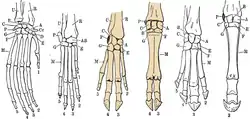
Anatomy

Artiodactyls are generally quadrupeds. Two major body types are known: Suinids and hippopotamuses are characterized by a stocky body, short legs, and a large head; camels and ruminants, though, have a more slender build and lanky legs. Size varies considerably; the smallest member, the mouse deer, often reaches a body length of only 45 centimeters (18 in) and a weight of 1.5 kilograms (3.3 lb). The largest member, the hippopotamus, can grow up to 5 meters (16 ft) in length and weigh 4.5 metric tons (5 short tons), and the giraffe can grow to be 5.5 meters (18 ft) tall and 4.7 meters (15 ft) in body length. All even-toed ungulates display some form of sexual dimorphism: the males are consistently larger and heavier than the females. In deer, only the males boast antlers, and the horns of bovines are usually small or not present in females. Male Indian antelopes have a much darker coat than females.
Almost all even-toed ungulates have fur, with an exception being the nearly hairless hippopotamus. Fur varies in length and coloration depending on the habitat. Species in cooler regions can shed their coat. Camouflaged coats come in colors of yellow, gray, brown, or black tones.
Limbs

Even-toed ungulates bear their name because they have an even number of toes (two or four)—in some peccaries, the hind legs have a reduction in the number of toes to three. The central axis of the leg is between the third and fourth toe. The first toe is missing in modern artiodactyls, and can only be found in now-extinct genera. The second and fifth toes are adapted differently between species:
 Hippopotamuses have all four toes pointing out.
Hippopotamuses have all four toes pointing out. For pigs and other biungulates the second and fifth toes are directed backwards.
For pigs and other biungulates the second and fifth toes are directed backwards. When camels have only two toes present, the claws are transformed into nails.
When camels have only two toes present, the claws are transformed into nails.
When camels have only two toes present, the claws are transformed into nails (while both are made of keratin, claws are curved and pointed while nails are flat and dull).[24] These claws consist of three parts: the plate (top and sides), the sole (bottom), and the bale (rear). In general, the claws of the forelegs are wider and blunter than those of the hind legs, and the gape is farther apart. Aside from camels, all even-toed ungulates put just the tip of the foremost phalanx on the ground.[25]
In even-toed ungulates, the bones of the stylopodium (upper arm or thigh bone) and zygopodiums (tibia and fibula) are usually elongated. The muscles of the limbs are predominantly localized, which ensures that artiodactyls often have very slender legs. A clavicle is never present, and the scapula is very agile and swings back and forth for added mobility when running. The special construction of the legs causes the legs to be unable to rotate, which allows for greater stability when running at high speeds. In addition, many smaller artiodactyls have a very flexible body, contributing to their speed by increasing their stride length.
Head
Many even-toed ungulates have a relatively large head. The skull is elongated and rather narrow; the frontal bone is enlarged near the back and displaces the parietal bone, which forms only part of the side of the cranium (especially in ruminants).
Horns and antlers

Four families of even-toed ungulates have cranial appendages. These Pecora (with the exception of the musk deer), have one of four types of cranial appendages: true horns, antlers, ossicones, or pronghorns.[26]
True horns have a bone core that is covered in a permanent sheath of keratin, and are found only in the bovids. Antlers are bony structures that are shed and replaced each year; they are found in deer (members of the family Cervidae). They grow from a permanent outgrowth of the frontal bone called the pedicle and can be branched, as in the white-tailed deer (Odocoileus virginianus), or palmate, as in the moose (Alces alces). Ossicones are permanent bone structures that fuse to the frontal or parietal bones during an animal's life and are found only in the Giraffidae. Pronghorns, while similar to horns in that they have keratinous sheaths covering permanent bone cores, are deciduous.[27]
All these cranial appendages can serve for posturing, battling for mating privilege, and for defense. In almost all cases, they are sexually dimorphic, and often found only on the males.
Teeth
| Dental formula | I | C | P | M | |
|---|---|---|---|---|---|
| 30–44 | = | 0–3 | 0–1 | 2–4 | 3 |
| 1–3 | 1 | 2–4 | 3 | ||
There are two trends in terms of teeth within Artiodactyla. The Suina and hippopotamuses have a relatively large number of teeth (with some pigs having 44); their dentition is more adapted to a squeezing mastication, which is characteristic of omnivores. Camels and ruminants have fewer teeth; there is often a yawning diastema, a designated gap in the teeth where the molars are aligned for crushing plant matter.
The incisors are often reduced in ruminants, and are completely absent in the upper jaw. The canines are enlarged and tusk-like in the Suina, and are used for digging in the ground and for defense. In ruminants, the males' upper canines are enlarged and used as a weapon in certain species (mouse deer, musk deer, water deer); species with frontal weapons are usually missing the upper canines. The lower canines of ruminants resemble the incisors, so that these animals have eight uniform teeth in the frontal part of the lower jaw.
The molars of porcine have only a few bumps. In contrast, the camels and ruminants have bumps that are crescent-shaped cusps (selenodont).
Senses
Artiodactyls have a well-developed sense of smell and sense of hearing. Unlike many other mammals, they have a poor sense of sight—moving objects are much easier to see than stationary ones. Similar to many other prey animals, their eyes are on the sides of the head, giving them an almost panoramic view.
Digestive system
The ruminants (Ruminantia and Tylopoda) ruminate their food—they regurgitate and re-chew it. Ruminants' mouths often have additional salivary glands, and the oral mucosa is often heavily calloused to avoid injury from hard plant parts and to allow easier transport of roughly chewed food. Their stomachs are divided into three to four sections: the rumen, the reticulum, the omasum, and the abomasum.[28] After the food is ingested, it is mixed with saliva in the rumen and reticulum and separates into layers of solid versus liquid material. The solids lump together to form a bolus (also known as the cud); this is regurgitated by reticular contractions while the glottis is closed. When the bolus enters the mouth, the fluid is squeezed out with the tongue and re-swallowed. The bolus is chewed slowly to completely mix it with saliva and to break it down. Ingested food passes to the "fermentation chamber" (rumen and reticulum), where it is kept in continual motion by rhythmic contractions. Cellulytic microbes (bacteria, protozoa, and fungi) produce cellulase, which is needed to break down the cellulose found in plant material.[28] This form of digestion has two advantages: plants that are indigestible to other species can be digested and used, and the duration of the actual food consumption shortened; the animal spends only a short time out in the open with his head to the ground—rumination can take place later, in a sheltered area.[29]
Tylopoda (camels, llamas, and alpacas) and chevrotains have three-chambered stomachs, while the rest of Ruminantia have four-chambered stomachs. The handicap of a heavy digestive system has increased selective pressure towards limbs that allow the animal to quickly escape predators.[30] Most species within Suina have a simple two-chambered stomach that allows an omnivorous diet. The babirusa, however, is an herbivore,[28] and has extra maxillary teeth to allow for proper mastication of plant material. Most of the fermentation occurs with the help of cellulolytic microorganisms within the caecum of the large intestine. Peccaries have a complex stomach that contains four compartments.[29] Their fore stomach has fermentation carried out by microbes and has high levels of volatile fatty acid; it has been proposed that their complex fore stomach is a means to slow digestive passage and increase digestive efficiency.[29] Hippopotamuses have three-chambered stomachs and do not ruminate. They consume around 68 kilograms (150 lb) of grass and other plant matter each night. They may cover distances up to 32 kilometers (20 mi) to obtain food, which they digest with the help of microbes that produce cellulase. Their closest living relatives, the whales, are obligate carnivores.
Unlike other even-toed ungulates, pigs have a simple sack-shaped stomach.[28] Some artiodactyla, such as white-tailed deer, lack a gall bladder.[31]

 As with all ruminants, deer have such a multi-chambered stomach, which is used for better digesting plant food.
As with all ruminants, deer have such a multi-chambered stomach, which is used for better digesting plant food.

Genitourinary system
The penises of even-toed ungulates have an S-shape at rest and lie in a pocket under the skin on the belly. The corpora cavernosa is only slightly developed; and an erection mainly causes this curvature to extend, which leads to an extension, but not a thickening, of the penis. Cetaceans have similar penises.[32] In some even-toed ungulates, the penis contains a structure called the urethral process.[33][34][35]
The testicles are located in the scrotum and thus outside the abdominal cavity. The ovaries of many females descend—as testicles descend of many male mammals—and are close to the pelvic inlet at the level of the fourth lumbar vertebra. The uterus has two horns (uterus bicornis).[32]
Other
The number of mammary glands is variable and correlates, as in all mammals, with litter size. Pigs, which have the largest litter size of all even-toed ungulates, have two rows of teats lined from the armpit to the groin area. In most cases, however, even-toed ungulates have only one or two pairs of teats. In some species, these form an udder in the groin region.
Secretory glands in the skin are present in virtually all species and can be located in different places, such as in the eyes; behind the horns, the neck, or back; on the feet; or in the anal region.
Lifestyle
Distribution and habitat
Artiodactyls are native to almost all parts of the world, with the exception of Oceania and Antarctica. Humans have introduced different artiodactyls worldwide as hunting animals.[36] Artiodactyls inhabit almost every habitat, from tropical rainforests and steppes to deserts and high mountain regions. The greatest biodiversity prevails in open habitats such as grasslands and open forests.
Social behavior
The social behavior of even-toed ungulates varies from species to species. Generally, there is a tendency to merge into larger groups, but some live alone or in pairs. Species living in groups often have a hierarchy, both among males and females. Some species also live in harem groups, with one male, several females, and their common offspring. In other species, the females and juveniles stay together, while males are solitary or live in bachelor groups and seek out females only during mating season.
Many artiodactyls are territorial and mark their territory, for example, with glandular secretions or urine. In addition to year-round sedentary species, there are animals that migrate seasonally.
There are diurnal, crepuscular, and nocturnal artiodactyls. Some species' pattern of wakefulness varies with season or habitat.
Reproduction and life expectancy

Generally, even-toed ungulates tend to have long gestation periods, smaller litter sizes, and more highly developed newborns. As with many other mammals, species in temperate or polar regions have a fixed mating season, while those in tropical areas breed year-round. They carry out polygynous mating behavior, meaning a male mates with several females and suppresses all competition.
The length of the gestation period varies from four to five months for porcine, deer, and musk deer; six to ten months for hippos, deer, and bovines; ten to thirteen months with camels; and fourteen to fifteen months with giraffes. Most deliver one or two babies, but some pigs can deliver up to ten.
The newborns are precocial (born relatively mature) and come with open eyes and are hairy (with the exception of the hairless hippos). Juvenile deer and pigs have striped or spotted coats; the pattern disappears as they grow older. The juveniles of some species spend their first weeks with their mother in a safe location, where others may be running and following the herd within a few hours or days.
The life expectancy is typically twenty to thirty years; as in many mammals, smaller species often have a shorter lifespan than larger species. The artiodactyls with the longest lifespans are the hippos, cows, and camels, which can live 40 to 50 years.
Predators and parasites
Artiodactyls have different natural predators depending on their size and habitat. There are several carnivores that would prey on such animals, including as large cats (e.g., lions) and bears. Other predators are crocodiles, wolves, large raptors, and for small species and young animals, large snakes.
Parasites include nematodes, botflies, fleas, lice, or flukes, but they have debilitating effects only when the infestation is severe.
Interactions with humans
Domestication

Artiodactyls have been hunted by primitive humans for various reasons: for meat or fur, as well as to use their bones and teeth as weapons or tools. Their domestication began around 8000 BCE. To date, humans have domesticated goats, sheep, cattle, camels, llamas, alpacas, and pigs. Initially, livestock was used primarily for food, but they began being used for work activities around 3000 BCE.[30] Clear evidence exists of antelope being used for food 2 million years ago in the Olduvai Gorge, part of the Great Rift Valley.[30][37] Cro-Magnons relied heavily on reindeer for food, skins, tools, and weapons; with dropping temperatures and increased reindeer numbers at the end of the Pleistocene, they became the prey of choice. Reindeer remains accounted for 94% of bones and teeth found in a cave above the Céou River that was inhabited around 12,500 years ago.[38]
Today, artiodactyls are kept primarily for their meat, milk, and wool, fur, or hide for clothing. Domestic cattle, the water buffalo, the yak, and camels are used for work, as rides, or as pack animals.[39]
Threats

The endangerment level of each even-toed ungulate is different. Some species are synanthropic (such as the wild boar) and have spread into areas that they are not indigenous to, either having been brought as farm animals or having run away as people's pets. Some artiodactyls also benefit from the fact that their predators (e.g. the Tasmanian tiger) were severely decimated by ranchers, who saw them as competition.[36]
Conversely, many artiodactyls have declined significantly in numbers, and some have even gone extinct, largely due to over-hunting, and, more recently, habitat destruction. Extinct species include several gazelles, the aurochs, the Malagasy hippopotamus, the bluebuck, and Schomburgk's deer. Two species, the Scimitar-horned oryx and Père David's deer, are extinct in the wild. Fourteen species are considered critically endangered, including the addax, the kouprey, the Bactrian camel, Przewalski's gazelle, the saiga, and the pygmy hog. Twenty-four species are considered endangered.[40][41]
See also
 Mammals portal
Mammals portal
References
- Jessica M Theodor; Jörg Erfurt; Grégoire Métais (23 October 2007). "The earliest artiodactyls: Diacodexeidae, Dichobunidae, Homacodontidae, Leptochoeridae and Raoellidae". In Donald R. Prothero; Scott E. Foss (eds.). Evolution of Artiodactyls. Johns Hopkins University. pp. 32–58. ISBN 9780801887352.
- Spaulding, M; O'Leary, MA; Gatesy, J (2009). Farke, Andrew Allen (ed.). "Relationships of Cetacea (Artiodactyla) Among Mammals: Increased Taxon Sampling Alters Interpretations of Key Fossils and Character Evolution". PLOS ONE. 4 (9): e7062. Bibcode:2009PLoSO...4.7062S. doi:10.1371/journal.pone.0007062. PMC 2740860. PMID 19774069.
- Montgelard, Claudine; Catzeflis, Francois M.; Douzery, Emmanuel (1997). "Phylogenetic relationships of artiodactyls and cetaceans as deduced from the comparison of cytochrome b and 12S rRNA mitochondrial sequences" (PDF). Molecular Biology and Evolution. 14 (5): 550–559. doi:10.1093/oxfordjournals.molbev.a025792. PMID 9159933.
- Groves, Colin P.; Grubb, Peter (2011). Ungulate Taxonomy. Baltimore, Maryland: Johns Hopkins University Press. p. 25. ISBN 978-1-4214-0093-8.
- Graur, Dan; Higgins, Desmond G. (1994). "Molecular Evidence for the Inclusion of Cetaceans within the Order Artiodactyla" (PDF). Molecular Biology and Evolution: 357–364. Archived from the original (PDF) on 5 March 2016. Retrieved 23 August 2015.
- Gatesy, John; Hayashi, Cheryl; Cronin, Mathew A.; Arctander, Peter (1996). "Evidence from milk casein genes that cetaceans are close relatives of hippopotamid artiodactyls". Molecular Biology and Evolution. 13 (7): 954–963. doi:10.1093/oxfordjournals.molbev.a025663. PMID 8752004.
- Shimamura, M. (1997). "Molecular evidence from retroposons that whales form a clade within even-toed ungulates". Nature. 388 (6643): 666–670. doi:10.1038/41759. PMID 9262399. S2CID 4429657.

- Gatesy, John (1997). "More DNA Support for a Cetacea/Hippopotamidae Clade: The Blood-Clotting Protein Gene y-Fibrinogen". Molecular Biology and Evolution. 14 (5): 537–543. doi:10.1093/oxfordjournals.molbev.a025790. PMID 9159931.
- Agnarsson, Ingi; May-Collado, Laura J. (2008). "The phylogeny of Cetartiodactyla: The importance of dense taxon sampling, missing data, and the remarkable promise of cytochrome b to provide reliable species-level phylogenies". Molecular Phylogenetics and Evolution. 48 (3): 964–85. doi:10.1016/j.ympev.2008.05.046. PMID 18590827.
- Gatesy, John; Milinkovitch, Michel; Waddell, Victor; Stanhope, Michael (1999). "Stability of Cladistic Relationships between Cetacea and Higher-Level Artiodactyl Taxa". Systematic Biology. 48 (1): 6–20. doi:10.1080/106351599260409. PMID 12078645.
- Madsen, Ole; Willemsen, Diederik; Ursing, Björn M.; Arnason, Ulfur; de Jong, Wilfried W. (2002). "Molecular Evolution of the Mammalian Alpha 2B Adrenergic Receptor". Molecular Biology and Evolution. 19 (12): 2150–2160. doi:10.1093/oxfordjournals.molbev.a004040. PMID 12446807.
- Amrine-Madsen, Heather; Koepfli, Klaus-Peter; Wayne, Robert K.; Springer, Mark S. (2003). "A new phylogenetic marker, apolipoprotein B, provides compelling evidence for eutherian relationships". Molecular Phylogenetics and Evolution. 28 (2): 225–240. doi:10.1016/s1055-7903(03)00118-0. PMID 12878460.
- Savage, R. J. G.; Long, M. R. (1986). Mammal Evolution: an illustrated guide. New York: Facts on File. pp. 208. ISBN 978-0-8160-1194-0.
- Price, Samantha A.; Bininda-Emonds, Olaf R. P.; Gittleman, John L. (2005). "A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla)". Biological Reviews. 80 (3): 445–73. doi:10.1017/s1464793105006743. PMID 16094808. S2CID 45056197.
- etwa noch bei Nowak (1999) oder Hendrichs (2004)
- Malcolm C. McKenna; Susan K. Bell (1997). 'Classification of Mammals - Above the Species Level. Columbia University Press. ISBN 978-0-231-11013-6.
- Beck, N.R. (2006). "A higher-level MRP supertree of placental mammals". BMC Evol Biol. 6: 93. doi:10.1186/1471-2148-6-93. PMC 1654192. PMID 17101039.
- O'Leary, M.A.; Bloch, J.I.; Flynn, J.J.; Gaudin, T.J.; Giallombardo, A.; Giannini, N.P.; Goldberg, S.L.; Kraatz, B.P.; Luo, Z.-X.; Meng, J.; Ni, X.; Novacek, M.J.; Perini, F.A.; Randall, Z.S.; Rougier, G.W.; Sargis, E.J.; Silcox, M.T.; Simmons, N.B.; Spaulding, M.; Velazco, P.M.; Weksler, M.; Wible, J.R.; Cirranello, A.L. (2013). "The Placental Mammal Ancestor and the Post-K-Pg Radiation of Placentals". Science. 339 (6120): 662–667. doi:10.1126/science.1229237. hdl:11336/7302. PMID 23393258. S2CID 206544776.
- Song, S.; Liu, L.; Edwards, S.V.; Wu, S. (2012). "Resolving conflict in eutherian mammal phylogeny using phylogenomics and the multispecies coalescent model". Proceedings of the National Academy of Sciences. 109 (37): 14942–14947. doi:10.1073/pnas.1211733109. PMID 22930817.
- dos Reis, M.; Inoue, J.; Hasegawa, M.; Asher, R.J.; Donoghue, P.C.J.; Yang, Z. (2012). "Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny". Proceedings of the Royal Society B: Biological Sciences. 279 (1742): 3491–3500. doi:10.1098/rspb.2012.0683. PMC 3396900. PMID 22628470.
- Upham, N.S.; Esselstyn, J.A.; Jetz, W. (2019). "Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation". PLOS Biology. 17 (12): e3000494. doi:10.1371/journal.pbio.3000494. PMID 31800571.(see e.g. Fig S10)
- Wilson, D. E.; Reeder, D. M., eds. (2005). Mammal Species of the World (3rd ed.). Johns Hopkins University Press. pp. 111–184. ISBN 978-0-8018-8221-0.
- Cui, P.; Ji, R.; Ding, F.; Qi, D.; Gao, H.; Meng, H.; Yu, J.; Hu, S.; Zhang, H. (2007). "A complete mitochondrial genome sequence of the wild two-humped camel (Camelus bactrianus ferus): an evolutionary history of Camelidae". BMC Genomics. 8 (1): 241. doi:10.1186/1471-2164-8-241. PMC 1939714. PMID 17640355.
- "Claws Out: Things You Didn't Know About Claws". Thomson Safaris. 7 January 2014. Retrieved 24 September 2016.
- Franz-Viktor Salomon (2008). musculoskeletal system. In: F.-V. Salomon and others (eds.): Anatomy for veterinary medicine. pp. 22–234. ISBN 978-3-8304-1075-1.
- DeMiguel, Daniel; Azanza, Beatriz; Morales, Jorge (2014). "Key innovations in ruminant evolution: a paleontological perspective". Integrative Zoology. 9 (4): 412–433. doi:10.1111/1749-4877.12080. PMID 24148672.
- Janis, C.M.; Scott, K.M. (1987). "The Interrelationships of Higher Ruminant Families with Special Emphasis on the Members of the Cervoidea". American Museum Novitates. 2893: 1–85. hdl:2246/5180.
- Janis, C.; Jarman, P. (1984). Macdonald, D. (ed.). The Encyclopedia of Mammals. New York: Facts on File. pp. 498–499. ISBN 978-0-87196-871-5.
- Shively, C. L.; et al. (1985). "Some Aspects of the Nutritional Biology of the Collared Peccary". The Journal of Wildlife Management. 49 (3): 729–732. doi:10.2307/3801702. JSTOR 3801702.
- "Artiodactyl". Encyclopædia Britannica Online. Encyclopædia Britannica, Inc. 2008. Retrieved 17 October 2008.
- Hewitt, David G (24 June 2011). Biology and Management of White-tailed Deer. ISBN 9781482295986.
- Uwe Gille (2008). urinary and sexual apparatus, urogenital Apparatus. In: F.-V. Salomon and others (eds.): Anatomy for veterinary medicine. pp. 368–403. ISBN 978-3-8304-1075-1.
- Spinage, C. A. "Reproduction in the Uganda defassa waterbuck, Kobus defassa ugandae Neumann." Journal of reproduction and fertility 18.3 (1969): 445-457.
- Yong, Hwan-Yul. "Reproductive System of Giraffe (Giraffa camelopardalis)." Journal of Embryo Transfer 24.4 (2009): 293-295.
- Sumar, Julio. "Reproductive physiology in South American Camelids." Genetics of Reproduction in Sheep (2013): 81.
- Pough, F. W.; Janis, C. M.; Heiser, J. B. (2005) [1979]. "Major Lineages of Mammals". Vertebrate Life (7th ed.). Pearson. p. 539. ISBN 978-0-13-127836-3.
- McKie, Robin; Editor, Science (22 September 2012). "Humans hunted for meat 2 million years ago". The Guardian. Retrieved 26 October 2015.CS1 maint: extra text: authors list (link)
- "Bones From French Cave Show Neanderthals, Cro-Magnon Hunted Same Prey". ScienceDaily. 2003. Retrieved 17 October 2008.
- Clay, J. (2004). World Agriculture and the Environment: A Commodity-by-Commodity Guide to Impacts and Practices. Washington, D.C., USA: Island Press. ISBN 978-1-55963-370-3.
- "Cetartiodactyla". Retrieved 12 March 2007.
- "Artiodactyla". Encyclopedia of Life. Retrieved 15 November 2014.
External links
| Wikimedia Commons has media related to Artiodactyla. |
| Wikispecies has information related to Artiodactyla. |