Iridium trihydride
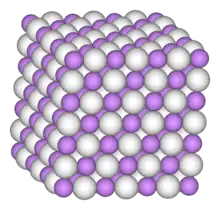
Iridium trihydride (IrH3) is a chemical compound of iridium and hydrogen that can be formed under high pressure. The crystalline form has a distorted simple cubic structure. The hydrogen atoms are on the centre of the faces of the crystal cell cube, and Iridium is at the centre. It forms at over 55 GPa.[1]
| Names | |
|---|---|
| IUPAC name
Iridium(III) hydride | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| H3Ir | |
| Molar mass | 195.241 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The bulk modulus of iridium trihydride is 190 GPa which is much less than that of iridium (383 GPa).
Decomposition of iridium trihydride is slow when the pressure is reduced to 6 GPa, and perhaps it can be metastable at atmospheric pressures.
A dihydride, IrH2, is predicted to be stable over 14 GPa.[2]
References
- Scheler, Thomas; Marqués, Miriam; Konôpková, Zuzana; Guillaume, Christophe L.; Howie, Ross T.; Gregoryanz, Eugene (19 November 2013). "High-Pressure Synthesis and Characterization of Iridium Trihydride" (PDF). Physical Review Letters. 111 (21): 215503. doi:10.1103/PhysRevLett.111.215503. PMID 24313503.
- Zaleski-Ejgierd, Patryk (2014). "High-pressure formation and stabilization of binary iridium hydrides". Physical Chemistry Chemical Physics. 16 (7): 3220–9. arXiv:1308.6724. doi:10.1039/C3CP54300E. PMID 24406641. S2CID 39756803.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.