Stannane
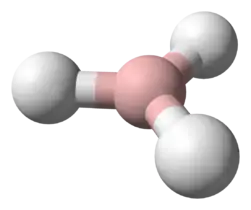
Stannane /ˈstæneɪn/ or tin hydride is an inorganic compound with the chemical formula SnH
4. It is a colourless gas and the tin analogue of methane. Stannane can be prepared by the reaction of SnCl4 and LiAlH4.[1]
- SnCl4 + LiAlH4 → SnH4 + LiCl + AlCl3
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Stannane | |||
| Other names
tin tetrahydride tin hydride stannane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| SnH4 | |||
| Molar mass | 122.71 g mol−1 | ||
| Appearance | colourless gas | ||
| Density | 5.4 g dm−3, gas | ||
| Melting point | −146 °C (−231 °F; 127 K) | ||
| Boiling point | −52 °C (−62 °F; 221 K) | ||
| Related compounds | |||
Related organotins |
tributylstannane (Bu3SnH) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Stannane decomposes slowly at room temperature to give metallic tin and hydrogen and ignites on contact with air.[1]
Variants of stannane can be found as a highly toxic, gaseous, inorganic metal hydride and group 14 hydride.
See also
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.