Lanthanum decahydride
Lanthanum decahydride is a polyhydride or superhydride compound of lanthanum and hydrogen (LaH10) that shows promise of being a high-temperature superconductor. It has a superconducting transition temperature TC ~ 250 K (−23 °C; −10 °F) at a pressure of 150 gigapascals (GPa), and its synthesis required pressures above ~160 GPa.[2] A cubic form can be synthezised at 1,000 K (730 °C; 1,340 °F),[2] and a hexagonal crystal structure can be formed at room temperature.[3]
| Identifiers | |
|---|---|
| Properties | |
| H10La | |
| Molar mass | 148.985 g·mol−1 |
| Structure[2] | |
| Cubic | |
| Fm3m | |
a = 5.1019(5) Å at 150 GPa | |
Lattice volume (V) |
132.80(4) Å3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
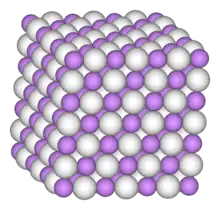
The cubic form has each lanthanum atom surrounded by 32 hydrogen atoms, which form the vertices of an 18 faced shape called a chamfered cube.[4]
References
- "Lanthanum decahydride". American Chemical Society.
- Drozdov, A. P.; Kong, P. P.; Minkov, V. S.; Besedin, S. P.; Kuzovnikov, M. A.; Mozaffari, S.; Balicas, L.; Balakirev, F. F.; Graf, D. E.; Prakapenka, V. B.; Greenberg, E.; Knyazev, D. A.; Tkacz, M.; Eremets, M. I. (2019). "Superconductivity at 250 K in lanthanum hydride under high pressures". Nature. 569 (7757): 528–531. arXiv:1812.01561. doi:10.1038/s41586-019-1201-8. PMID 31118520. S2CID 119231000.
- Geballe, Zachary M.; Liu, Hanyu; Mishra, Ajay K.; Ahart, Muhtar; Somayazulu, Maddury; Meng, Yue; Baldini, Maria; Hemley, Russell J. (15 January 2018). "Synthesis and Stability of Lanthanum Superhydrides". Angewandte Chemie International Edition. 57 (3): 688–692. doi:10.1002/anie.201709970. PMID 29193506.
- "NNNS chemistry blog: Lanthanum decahydride". chem.vander-lingen.nl.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.