Lymphangioleiomyomatosis
Lymphangioleiomyomatosis (LAM) is a rare, progressive and systemic disease that typically results in cystic lung destruction. It predominantly affects women, especially during childbearing years.[1] The term sporadic LAM is used for patients with LAM not associated with tuberous sclerosis complex (TSC), while TSC-LAM refers to LAM that is associated with TSC.[2]
| Lymphangioleiomyomatosis (LAM) | |
|---|---|
| Other names | lymphangiomyomatosis, LAM |
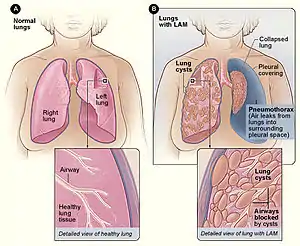 | |
| Figure A shows the location of the lungs and airways in the body. The inset image shows a cross-section of a healthy lung. Figure B shows a view of the lungs with LAM and a collapsed lung (pneumothorax). The inset image shows a cross-section of a lung with LAM. | |
| Specialty | Pulmonology |
Signs and symptoms
The average age of onset is the early to mid 30s.[3][4][5][6] Exertional dyspnea (shortness of breath) and spontaneous pneumothorax (lung collapse) have been reported as the initial presentation of the disease in 49% and 46% of patients, respectively.[6]
Diagnosis is typically delayed 5 to 6 years.[3][4][5][6] The condition is often misdiagnosed as asthma or chronic obstructive pulmonary disease. The first pneumothorax, or lung collapse, precedes the diagnosis of LAM in 82% of patients.[7][8] The consensus clinical definition of LAM includes multiple symptoms:
- Fatigue
- Cough
- Coughing up blood (rarely massive)
- Chest pain
- Chylous complications arising from lymphatic obstruction, including
- Chylothorax
- Chylous ascites
- Chylopericardium
- Chyloptysis
- Chyluria
- Chyle in vaginal discharge
- Chyle in stool.
- Angiomyolipomas (fatty kidney tumors) are present in about 30% of patients with sporadic LAM and up to 90% of patients with TSC-LAM.[9][10] Angiomyolipomas can sometimes spontaneously bleed, causing pain or low blood pressure.
- Cystic lymphangiomas or lymph nodes with hypodense centers, which mimic necrotizing lymphomas, ovarian or renal cancers, or other malignancies can occur in the retroperitoneum, pelvis or mediastinum.[11][12][13][14]
Lung destruction in LAM is a consequence of diffuse infiltration by neoplastic smooth muscle-like cells that invade all lung structures including the lymphatics, airway walls, blood vessels and interstitial spaces.[15] The consequences of vessel and airway obstruction include chylous fluid accumulations, hemoptysis, airflow obstruction and pneumothorax. The typical disease course displays progressive dyspnea on exertion, spaced by recurrent pneumothoraces and in some patients, chylous pleural effusions or ascites.[16]
Most people have dyspnea on exertion with daily activities by 10 years after symptom onset. Many patients require supplemental oxygen over that interval.[7]
Genetics
LAM occurs in two settings: in the disease tuberous sclerosis complex (TSC-LAM) and in a sporadic form, in women who do not have TSC (sporadic LAM).[17][18] In both settings, genetic evidence indicates that LAM is caused by inactivating or “loss of function” mutations in the TSC1 or TSC2 genes, which were cloned in 1997 and 1993 respectively.[19] The TSC1 gene is located on the long arm of chromosome 9 (9q34) and the TSC2 gene is located on the short arm of chromosome 16 (16p13). TSC-LAM occurs in women who have germline mutations in either the TSC1 or the TSC2 gene.[20]
Sporadic LAM is primarily associated with somatic TSC2 gene mutations.[21][22] Germline and somatic mutations in LAM include many types of mutations spread across the genes, with no clear “hot spots,” including missense changes, in-frame deletions and nonsense mutations.[20][21][22] Because of the large size of the genes (together they have more than 60 exons) and because mutations can be located virtually anywhere within the genes, mutation detection is often challenging.
On a cellular basis, LAM cells carry bi-allelic inactivation of the TSC2 genes, consistent with the “two-hit” tumor suppressor gene model.[23][24] The second hit event in LAM cells is often loss of the chromosomal region containing the wild-type copy of the TSC2 gene; this is referred to as loss of heterozygosity or LOH.[25] LOH can be detected in microdissected LAM cells,[21][26] in angiomyolipomas and lymph nodes from women with LAM,[27] and in circulating LAM cells (cells in blood and urine).[28][29]
Angiomyolipomas and pulmonary LAM cells from women with the sporadic form of LAM carry identical mutations in TSC2.[21] This, together with the fact that recurrent LAM after lung transplantation carries the same TSC2 mutations as the original LAM,[30] has led to the "benign metastasis" hypothesis that LAM cells can migrate or metastasize from one site to another.[17][18]
Pathophysiology
A variable percentage of cells within the LAM lesion contain mutational inactivation of the Tuberous Sclerosis Complex (TSC1 or TSC2) tumor suppressor genes.[21][27][31] TSC1 mutations cause a less severe clinical phenotype than TSC2 mutations.[32] The discovery of TSC1/2 gene function as negative regulator of the mammalian target of rapamycin complex 1 (mTORC1)[33][34] led to successful use of rapamycin analog sirolimus in clinical trials[35][36] and FDA approval of sirolimus for treatment of LAM.
TSC1 and TSC2 form a tumor suppressor complex that regulates mammalian target of rapamycin (mTOR) signaling complex by directly controlling the activity of the small GTPase Rheb via the GTPase activating protein (GAP) domain of TSC2. Rheb binds to Raptor and controls the activity of mTOR complex 1 (mTORC1) that directly phosphorylates p70 S6 kinase (S6K1) and 4E-BP1. mTOR forms two physically and functionally distinct multiprotein complexes: the rapamycin-sensitive mTORC1 and the rapamycin-insensitive mTORC2.[37] MTORC1 consists of five proteins including Raptor that positively regulate mTOR activity.[38][39][40] MTORC2 consists of six proteins including mTOR and Rictor, which defines the activation level of mTORC2[41][42][43] and modulates the assembly of the actin cytoskeleton through Rho GTPases,[44][45][46] and Rac1 is required for mTOR activation.[47] In TSC2-null and human LAM cells, Rho GTPase activity is required for cell adhesion, motility, proliferation and survival.[48][49][50] Loss of TSC1/TSC2 in LAM induces uncontrolled LAM cell growth and increases LAM cell viability. Upregulation of STAT1 and STAT3[51][52][53][54] and autophagy[55] are known mediators of LAM cell viability and survival.
LAM cells behave, in many ways, like metastatic tumor cells.[56] LAM cells appear to arise from an extrapulmonary source and migrate to the lung.[21] Increased LAM cell migration and invasiveness is rescued by TSC2 re-expression.[49] The cellular and molecular mechanisms of neoplastic transformation and lung parenchymal destruction by LAM cells remain unknown. Lung remodeling may be mediated by an imbalance between matrix degrading metalloproteinases (MMPs) and their endogenous inhibitors TIMPs.[57] The invasive cell phenotype in LAM is associated with TIMP-3 downregulation[58] and TSC2-dependent upregulation of MMPs.[59][60][61][62]
Clinical and histopathological evidence demonstrate the lymphatic involvement in LAM.[14][57][63][64][65][66][67][68] The prevailing hypothesis is that LAM lesions secrete the lymphangiogenic factor VEGF-D, recruit lymphatic endothelial cells (LECs) that form lymphatic vessels and induce lung cysts.[57] VEGF-D serum levels are increased in LAM[69] compared to other cystic lung diseases, including pulmonary Langerhans cell histiocytosis, emphysema, Sjögren's syndrome, or Birt–Hogg–Dubé syndrome.[70] VEGF-D levels correlate with the severity of LAM, evaluated as a measure of CT grade (the abundance of chylous effusions and lymphatic involvement).[71] VEGF-D is a secreted homodimeric glycoprotein and a member of the VEGF family of growth factors, is known for its role in cancer lymphangiogenesis and metastasis.[72][73][74] Proteolytic processing of VEGF-D affects cognate binding to VEGFR3.[75] Histopathologically, LAM lesions are surrounded by cells that stain for VEGFR3, the lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) and podoplanin.[63][76] VEGF-D binds to the receptor protein tyrosine kinases VEGFR-2 and VEGFR-349 in humans, and to VEGFR3 in mice.[74][77] Surprisingly, knock-out of VEGF-D in mice has little effect on lymphatic system development.[78] Nevertheless, during tumorigenesis VEGF-D promotes formation of tumor lymphatic vessels and facilitates metastatic spread of cancer cells.[73][74] However, little is known about a role of abnormal lymphatics and VEGF-D in LAM pathogenesis.
Diagnosis
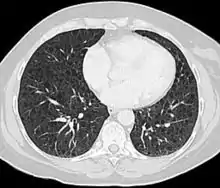
LAM can come to medical attention in several ways, most of which trigger a chest CT. Thin-walled cystic change in the lungs may be found incidentally on CT scans of the heart, chest or abdomen (on the cuts that include lung bases) obtained for other purposes. HRCTs of TSC patients reveals that about 20% of women have cystic change by age 20 and about 80% of women have cystic changes after age 40.[79] LAM is sometimes revealed by chest CT in patients who present with an apparent primary spontaneous pneumothorax, but more often CT scanning is not ordered (in the United States) until recurrences occur. Progressive dyspnea on exertion without the exacerbations and remissions that are characteristic of asthma or COPD sometimes prompt a chest CT. A review of the CT by an expert familiar with LAM may increase diagnostic accuracy.[80] Chylothorax can also bring LAM to attention.
In some cases, a LAM diagnosis can be made with confidence on clinical grounds (without biopsy) in patients with typical cystic changes on high resolution CT scanning of the lung and findings of tuberous sclerosis, angiomyolipoma, lymphangioleiomyoma, chylothorax or serum VEGF-D > 800 pg/ml.[70][81]
If none of these clinical features are present, a biopsy may be necessary to make the diagnosis. Video-assisted thoracoscopic lung biopsy is the most definitive technique, but transbronchial biopsy has a yield of over 50% and can also be effective.[82][83] The safety of the latter procedure in patients with diffuse cystic disease and the profusion of cystic change that predicts an informative biopsy are incompletely understood, however. Cytology of chylous fluids, aspirated abdominal nodes or lymphatic masses can also be diagnostic.[63][84][85][86]
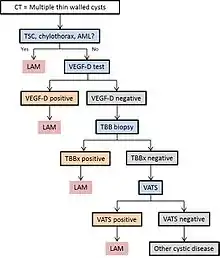
Diagram 1 outlines a proposed algorithm for the diagnosis of LAM.
Chest radiograph
The chest radiograph may appear relatively normal, even late in the disease, or may suggest hyperinflation only. As the disease progresses, the chest radiograph often demonstrates diffuse, bilateral and symmetric reticulonodular opacities, cysts, bullae or a "honeycomb" (i.e., pseudo fibrotic) appearance.[3][6] Pleural effusion and pneumothorax may be apparent. Preservation of lung volumes in the presence of increased interstitial markings is a radiographic hallmark of LAM that helps distinguish it from most other interstitial lung diseases, in which alveolar septal and interstitial expansion tend to increase the lung's elastic recoil properties and decreased lung volumes.
Computed tomography
The high-resolution computed tomography (HRCT) chest scan is better than the chest radiograph to detect cystic parenchymal disease and is almost always abnormal at the time of diagnosis, even when the chest radiograph and pulmonary function assessments are normal.[3][5][6][87] The typical CT shows diffuse round, bilateral, thin-walled cysts of varying sizes ranging from 1 to 45 mm in diameter.[5][6] The numbers of cysts varies in LAM from a few to almost complete replacement of normal lung tissue. The profusion of cysts tends to be milder in patients with TSC-LAM than S-LAM, perhaps explained in part because TSC-LAM patients typically receive earlier screening.[11] Pleural effusions are seen on CT in 12% of patients with S-LAM and 6% of patients with TSC-LAM. Other CT features include linear densities (29%), hilar or mediastinal lymphadenopathy (9%), pneumothorax, lymphangiomyoma, and thoracic duct dilation.[5][6] Ground-glass opacities (12%) suggest the presence of interstitial edema due to lymphatic congestion. In patients with TSC, nodular densities on HRCT may represent multifocal micronodular pneumocyte hyperplasia (MMPH) made up of clusters of hyperplastic type II pneumocytes.[79][88][89] MMPH may be present in males or females with TSC in the presence or absence of LAM, but not in patients with S-LAM.[90] MMPH is not typically associated with physiologic or prognostic consequences, but one case of respiratory failure due to MMPH has been reported.[91][92][93]
Ventilation-perfusion scans
In one study ventilation-perfusion scans were abnormal in 34 of 35 LAM patients.[5] The most common abnormality was nonspecific diffuse heterogeneity, usually grossly matched. These authors also described an “unusual,” “speckling pattern” on the perfusion images in 74% of patients, consisting of “small, often peripheral collections of radioisotope.”
Positron emission tomography
LAM and AML lesions do not typically exhibit increased uptake of 18F-fluorodeoxyglucose on positron emission tomography (PET) scanning.[94][95] Other neoplasms (or sources of inflammation) should therefore be considered in known or suspected LAM cases in which FDG-PET results are positive.[96]
Abdominal imaging
Abnormalities on abdominal imaging, such as renal AML and enlarged lymphatic structures, are also common in LAM. Fat density within a renal mass is pathognomonic of AMLs. AMLs are more prevalent and more frequently bilateral and large in patients with TSC-LAM than in patients with S-LAM. AML size correlates with the prevalence of pulmonary cysts in patients with TSC.[9] One study CT imaged 256 patients with S-LAM and 67 with TSC-LAM. Renal AMLs were present in 32% of patients with S-LAM and 93% of patients with TSC-LAM. Hepatic AMLs were present in 2% of patients with S-LAM and 33% of patients with TSC-LAM. Ascites was uncommon, seen in fewer than 10% of patients with LAM. Abdominal lymphangiomatosis, often containing both cystic and solid components, were seen in 29% of patients with S-LAM and 9% of patients with TSC-LAM.[11]
Central nervous system imaging
Central nervous system abnormalities, such as cortical or subependymal tubers and astrocytomas, are common in patients with TSC, including those with TSC-LAM, but are not found in women with S-LAM. Moss and associates[97] reported that women with S-LAM and TSC-LAM may have an increased incidence of meningioma, but the significance of that finding has been challenged.[98]
Pulmonary function studies
Pulmonary function testing in patients with LAM may be normal or may reveal obstructive, restrictive or mixed patterns. Obstructive physiology is the most common abnormality. Quality-controlled lung function data were collected prospectively by the NHLBI Registry, a 5-year study of patients with LAM in centers around the United States. Spirometry revealed obstructive changes in about 57% of patients and normal results in 34%.[10] Restriction, defined as a total lung capacity less than the lower limit of normal, was seen in 11%. Hyperinflation was present in about 6%. The average residual volume was 125% of predicted when measured by plethysmography, but was only 103% of predicted determined with gas dilution methods, suggesting significant air trapping in noncommunicating airspaces. Approximately 25% of patients with obstructive physiology may demonstrate bronchodilator responsiveness but may be less in more severe obstruction.[99][100] The obstructive physiologic defect in LAM is primarily attributable to airflow obstruction.[101] The earliest change in initial pulmonary function testing in various case series was abnormal gas transfer, as assessed by the diffusing capacity for carbon monoxide (DLCO), described in 82% to 97% of patients.[3][4][6] It is not unusual for DLCO to be reduced out of proportion to forced expiratory volume in 1 second (FEV1).[99] Reduction in DLCO and increase in residual volume are generally considered to be LAM's earliest physiologic manifestations.
Cardiopulmonary exercise testing in a much larger cohort of patients with LAM revealed a reduced maximal oxygen consumption (VO2 max) and anaerobic threshold in 217 patients.[102][103] Exercise-induced hypoxemia was found even in patients who did not have resting abnormalities in FEV1 and DLCO. In most patients, exercise was thought to be ventilation limited, owing to airflow obstruction and increased dead-space ventilation.
Disease progression is usually accompanied by a progressive obstructive ventilatory defect. Decline in FEV1 is the most commonly used parameter to monitor disease progression. Although resting pulmonary hypertension appears to be unusual in LAM, pulmonary arterial pressure often rises with low levels of exercise, related in part to hypoxemia.[103] One study reported an increase in intraparenchymal shunts in dyspneic patients with LAM, which may contribute to resting and exercise hypoxemia.[104]
Pathology
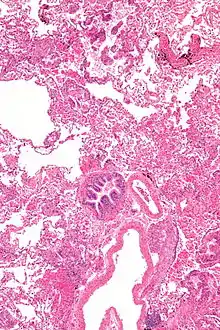
Grossly, LAM lungs are enlarged and diffusely cystic, with dilated air spaces as large as several centimeters in diameter.[105][106] Microscopic examination of the lung reveals foci of smooth muscle-like cell infiltration of the lung parenchyma, airways, lymphatics, and blood vessels associated with areas of thin-walled cystic change. LAM lesions often contain an abundance of lymphatic channels, forming an anastomosing meshwork of slit-like spaces lined by endothelial cells. LAM cells generally expand interstitial spaces without violating tissue planes but have been observed to invade the airways, the pulmonary artery, the diaphragm, aorta, and retroperitoneal fat, to destroy bronchial cartilage and arteriolar walls, and to occlude the lumen of pulmonary arterioles.[105]
There are two major cell morphologies in the LAM lesion: small spindle-shaped cells and cuboidal epithelioid cells.[107] LAM cells stain positively for smooth muscle actin, vimentin, desmin, and, often, estrogen and progesterone receptors. The cuboidal cells within LAM lesions also react with a monoclonal antibody called HMB-45, developed against the premelanosomal protein gp100, an enzyme in the melanogenesis pathway.[107] This immunohistochemical marker is very useful diagnostically, because other smooth muscle–predominant lesions in the lung do not react with the antibody.[108] The spindle-shaped cells of the LAM lesion are more frequently proliferating cell nuclear antigen positive than the cuboidal cells, consistent with a proliferative phenotype.[107] Compared with cigar-shaped normal smooth muscle cells, spindle-shaped LAM cells contain less abundant cytoplasm and are less eosinophilic. Estrogen and progesterone receptors are also present in LAM lesions,[109][110][111] but not in adjacent normal lung tissue.[112] LAM lesions express lymphatic markers LYVE-1, PROX1, podoplanin and VEGFR-3. The smooth muscle–like cells of AMLs are morphologically and immunohistochemically similar to LAM cells, including reactivity with antibodies directed against actin, desmin, vimentin, and HMB-45 as well as estrogen and progesterone receptors.[113][114] Unlike the dilated airspaces in emphysema, the cystic spaces found in LAM may be partially lined with hyperplastic type II cells.[115]
Treatment
An FDA-approved drug for treatment of LAM, the mTOR inhibitor sirolimus, is available for stabilization of lung function decline.[35] Lung transplant remains the last resort for patients with advanced disease.[116]
Pneumothorax
Pneumothoraces in LAM patients tend to recur, especially after conservative management such as observation, aspiration or simple tube thoracostomy. Over 65% of LAM patients develop pneumothorax during the course of their illness, averaging 3.5 pneumothoraces in those who have at least one pneumothorax.[8] The LAM Foundation Pleural Consensus Group advocated the use of a pleural symphysis procedure with the first pneumothorax, given the greater than 70% chance of recurrence.[8] Chemical sclerosis, mechanical abrasion, talc poudrage and pleurectomy have been effective in patients with LAM, but mechanical abrasion is preferred for those who may require pulmonary transplantation in the future. About half of LAM patients who have undergone transplant have had a prior pleurodesis procedure, and more than 75% of those had had prior bilateral pleurodesis.[8] Although pleurodesis is not a contraindication to transplantation, it can result in increased perioperative bleeding.
Chylothorax
Chyle does not generally cause pleural inflammation or fibrosis. Small stable chylous effusions rarely require intervention once the LAM diagnosis is made. Shortness of breath may mandate possibly repeated drainage. Sirolimus is effective for chylous effusions and most experts believe it should be used as the first line of therapy.[65] Imaging the source of the leak with heavy T2-weighted MRI or contrast lymphangiography is an advised for refractory effusions.[117] Some leaks are amenable to embolization through catheters threaded from groin lymph nodes into the thoracic duct. Thoracic duct ligation can be considered, but since thoracic effusions sometimes originate from ascites that are siphoned into the chest by the bellows action of the thorax, it is important to rule out an abdominal source before considering this option. Pleural symphysis may be required to prevent nutritional and lymphocyte deficiencies that can result from repeated taps or persistent drainage. Chemical pleurodesis is generally an effective therapy for chylothorax, as is mechanical abrasion and talc poudrage.[118]
Angiomyolipoma
Renal angiomyolipomas (AMLs) may require embolization or cauterization for control of bleeding, a complication that is thought to be more common when tumor diameter exceeds 4 cm.[119] The extent of aneurysmal change may determine bleeding risk. Serial abdominal imaging should be performed to assess AML size at 6- to 12-month intervals, at least until trends in growth are clear. Nephron sparing partial resections may be considered for very large tumors.[120] Nephrectomy is sometimes required for tumors with intravascular extension or other reasons, but is rarely the approach of choice for AMLs that can be managed by less invasive means. Everolimus is approved by the U.S. Food and Drug Administration (FDA) for AML treatment.[121]
Lymphangioleiomyoma
Lymphangioleiomyomatoses are fluid-filled hypodense structures present in the retroperitoneal regions of the abdomen and pelvis in about 30% of LAM patients. They generally do not require intervention. Biopsy or resection can lead to prolonged leakage. mTOR inhibitors are effective at shrinking the size of lymphangioleiomyomatosis, and can lead to total resolution.
Management-other
Estrogen-containing medications can exacerbate LAM[122] and are contraindicated. Agents that antagonize the effects of estrogen have not been proven to be effective for treatment, but no proper trials have been done. A trial of bronchodilators should be considered in LAM patients, because up to 17% to 25% have bronchodilator-responsive airflow obstruction.[5][10] Oxygen should be administered to maintain oxyhemoglobin saturations of greater than 90% with rest, exercise and sleep. Bone densitometry should be considered in all patients who are immobilized and/or on antiestrogen therapies, and appropriate therapy instituted for osteoporotic patients. Proper attention should be paid to cardiovascular health following natural or induced menopause. Immunizations for pneumococcus and influenza should be kept up to date. Pulmonary rehabilitation seems to be particularly rewarding in young, motivated patients with obstructive lung disease, but studies to assess this intervention's effect on exercise tolerance, conditioning and quality of life have not been done.
Medication
Sirolimus is an mTOR inhibitor that stabilizes lung function and improves some measures of life in LAM patients.[35] It is approved by the FDA for use in LAM, based on the results of the Multicenter International LAM Efficacy and Safety of Sirolimus (MILES) Trial. MILES data supports the use of sirolimus in patients who have abnormal lung function (i.e. FEV1<70% predicted). Whether the benefits of treatment outweigh the risks for asymptomatic LAM patients with normal lung function is not clear, but some physicians consider treatment for declining patients who are approaching the abnormal range for FEV1. Sirolimus also appears to be effective for the treatment chylous effusions and lymphangioleiomyomatosis. The benefits of sirolimus only persist while treatment continues. The safety of long term therapy has not been studied.
Potential side effects from mTOR inhibitors include swelling in the ankles, acne, oral ulcers, dyspepsia, diarrhea, elevation of cholesterol and triglycerides, hypertension and headache. Sirolimus pneumonitis and latent malignancy are more serious concerns, but occur infrequently. Sirolimus inhibits wound healing. It is important to stop therapy with the drug for 1–2 weeks before and after elective procedures that require optimal wound healing. Precautions must be taken to avoid prolonged sun exposure due to increased skin cancer risk.
Treatment with another mTOR inhibitor, everolimus, was reported in a small, open-label trial to be associated with improvement in FEV1 and six-minute walk distance.[123] Serum levels of VEGF-D and collagen IV were reduced by treatment. Adverse events were generally consistent with those known to be associated with mTOR inhibitors, although some were serious and included peripheral edema, pneumonia, cardiac failure and Pneumocystis jirovecii infection. Escalating doses of everolimus were used, up to 10 mg per day; higher than what is typically used clinically for LAM.
Serum VEGF-D concentration is useful, predictive and prognostic biomarker.[71] Higher baseline VEGF-D levels predicts more rapid disease progression and a more robust treatment response.
Hormonal approaches to treatment have never been tested in proper trials. In the absence of proven benefit, therapy with progesterone, GnRh agonists (e.g., leuprorelin, goserelin) and tamoxifen are not routinely recommended. Doxycycline had no effect on the rate of lung function decline in a double blind trial.[124]
Sirolimus is often effective as first-line management for chylothorax.[65] If chylous leakage or accumulations persist despite treatment, imaging with heavy T2 weighted MRI, MRI lymphangiography or thoracic duct lymphangiography can be considered. Pleural fusion procedures can be considered in refractory cases.
Prognosis
Survival estimates vary, dependent on mode of presentation or ascertainment, and have generally trended upward, probably due to earlier recognition through more widespread use of CT scanning. In a recent population-based cohort survey, median survival was found to be 29 years.[125] Data from earlier, large case series indicated that 38% to 78% of patients were alive at 8.5 years from the time of disease onset.[3][4][6][126]
Patients typically develop progressive airflow obstruction. In a cohort of patients in the United Kingdom, 10 years after symptom onset, 55% of 77 patients were breathless walking on flat ground and 10% were housebound.[127] The average annual rate of decline in FEV1 and DLCO in 275 patients studied in a single pulmonary function laboratory at the NHLBI was 75 ± 9 mL, and 0.69 ± 0.07 mL/min/mm Hg, respectively.[128] In other series from Europe, the rate of decline in FEV1 was considerably higher, estimated at approximately 100 to 120 mL/yr.[6][129][130] In the MILES trial, patients in the placebo group lost 134 cc/yr.[35] There was some evidence in these studies that rate of decline in lung function correlates with initial DLCO, with menopausal status and high baseline VEGF-D.
Estimates of median survival vary from 10 to 30 years, depending on whether hospital-based or population-based cohorts are studied.[98][125][131]
Epidemiology
LAM is almost completely restricted to women.[132][133] While lung cysts consistent with LAM are reported in some men with tuberous sclerosis, very few of these men develop symptoms. The prevalence of LAM is estimated using data from registries and patient groups and is between 3.4 and 7.8/million women. The number of new cases each year is between 0.23 and 0.31/million women/year in the US, UK and Switzerland. The variation between countries and between adjacent states in the US, suggest that a significant number of women with LAM remain either undiagnosed or their symptoms are attributed to other diseases.[134] Adult women with tuberous sclerosis are more likely to develop LAM than women without tuberous sclerosis. Cohorts of patients with tuberous sclerosis have been screened for LAM using CT scanning. In a retrospective study of adults with tuberous sclerosis, CT demonstrated lung cysts in 42% of 95 women and 13% of 91 men. In general, lung cysts were larger and more numerous in women than in men.[135] In a further retrospective study of women with TSC who underwent CT scanning to detect LAM, 25% of those in their 20s had lung cysts whereas 80% of women in their 40s were affected, suggesting that the development of LAM is age dependent at least in tuberous sclerosis-related LAM.[79] Although the prevalence of tuberous sclerosis at 1 in 6000 births is much greater than that of LAM, most pulmonary clinics see more cases of sporadic than tuberous sclerosis-LAM: probably due to a combination of low levels of screening for LAM in tuberous sclerosis and in many, the absence of symptoms.
Female sex and tuberous sclerosis are the only known risk factors. Although use of supplemental estrogen is not associated with development of LAM,[136] one study suggested that use of estrogen-containing contraceptive pills was associated with earlier onset.[137]
It occurs in more than 30% of women with tuberous sclerosis complex (TSC-LAM), a heritable syndrome that is associated with seizures, cognitive impairment and benign tumors in multiple tissues.[9][138][139][79] Most LAM patients who present for medical evaluation have the sporadic form of the disease (S-LAM), however, which is not associated with other manifestations of tuberous sclerosis complex.
Mild cystic changes consistent with LAM have been described in 10–15% of men with TSC,[140][135] but symptomatic LAM in males is rare.[132][133] Sporadic LAM occurs exclusively in women, with one published exception to date.[133] Both TSC-LAM and S-LAM are associated with mutations in tuberous sclerosis genes.[21]
Pregnancy
Pregnancy has been reported to exacerbate LAM in some cases.[96][141][142][143][144] However, the risk has not been rigorously studied. In a survey of 318 patients who indicated that they had had at least one pregnancy, 163 responded to a second survey focusing on lung collapse.[145] A total of 38 patients reported a pneumothorax with pregnancy, consistent with an incidence of pneumothorax in pregnancy of at least 10% (38 of 318). In one third of patients, the pneumothorax during pregnancy led to the LAM diagnosis. Pneumothoraces were almost twice as frequent on the right as on the left, and four women presented with bilateral spontaneous pneumothorax. Most pneumothoraces took place during the second and third trimesters. This study and others[7][6] suggest that pregnancy is associated with pleural complications in LAM patients. Few women with a known LAM diagnosis choose to become pregnant and patients in whom LAM is diagnosed during pregnancy rarely have baseline pulmonary function tests available, complicating resolution of this question.
Society
The LAM Foundation was founded in 1995 as a grassroots organization to provide patient advocacy and research funding.[146] Today, the LAM Foundation provides support and education for women with LAM and their families, engages doctors and scientists to continue to learn more about the disease, and raises funds for the continued study of LAM. It seeks safe and effective treatments, and ultimately a cure, for lymphangioleiomyomatosis. It is headquartered in Cincinnati, Ohio.
In popular culture
In "Lucky Thirteen", the fifth episode of the fifth season of House, Spencer (Angela Gots) was diagnosed with LAM, though later it was found to be a case of Sjögren's syndrome.
See also
References
- McCormack FX (February 2008). "Lymphangioleiomyomatosis: a clinical update". Chest. 133 (2): 507–16. doi:10.1378/chest.07-0898. PMID 18252917.
- "Sporadic lymphangioleiomyomatosis: Clinical presentation and diagnostic evaluation". UpToDate. Retrieved 19 March 2018.
- Kitaichi, M; Nishimura, K; Itoh, H; Izumi, T (1995). "Pulmonary lymphangioleiomyomatosis: A report of 46 patients including a clinicopathologic study of prognostic factors". Am J Respir Crit Care Med. 151 (2): 527–533. doi:10.1164/ajrccm.151.2.7842216. PMID 7842216.
- Taylor, JR; Ryu, J; Colby, TV; Raffin, TA (1990). "Lymphangioleiomyomatosis. Clinical course in 32 patients". N Engl J Med. 323 (18): 1254–1260. doi:10.1056/nejm199011013231807. PMID 2215609.
- Chu, SC; Horiba, K; Usuki, J; Avila, NA; Chen, CC; Travis, WD; Ferrans, VJ; Moss, J (1999). "Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis". Chest. 115 (4): 1041–1052. doi:10.1378/chest.115.4.1041. PMID 10208206.
- Urban, T; Lazor, R; Lacronique, J; Murris, M; Labrune, S; Valeyre, D; Cordier, JF (1999). "Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P)". Medicine (Baltimore). 78 (5): 321–337. doi:10.1097/00005792-199909000-00004. PMID 10499073.
- Johnson, SR; Tattersfield, AE (2000). "Clinical experience of lymphangioleiomyomatosis in the UK". Thorax. 55 (12): 1052–1057. doi:10.1136/thorax.55.12.1052. PMC 1745654. PMID 11083892.
- Almoosa, KF; Ryu, JH; Mendez, J; Huggins, JT; Young, LR; Sullivan, EJ; Maurer, J; McCormack, FX; Sahn, SA (2006). "Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications". Chest. 129 (5): 1274–1281. doi:10.1378/chest.129.5.1274. PMID 16685019.
- Franz, DN; Brody, A; Meyer, C; Leonard, J; Chuck, G; Dabora, S; Sethuraman, G; Colby, TV; Kwiatkowski, DJ; McCormack, FX (2001). "Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis". Am J Respir Crit Care Med. 164 (4): 661–668. doi:10.1164/ajrccm.164.4.2011025. PMID 11520734.
- Ryu, JH; Moss, J; Beck, GJ; Lee, JC; Brown, KK; Chapman, JT; Finlay, GA; Olson, EJ; Ruoss, SJ; Maurer, JR; Raffin, TA; Peavy, HH; McCarthy, K; Taveira-Dasilva, A; McCormack, FX; Avila, NA; Decastro, RM; Jacons, SS; Stylianou, M; Fanburg, BL (2006). "The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment". Am J Respir Crit Care Med. 173 (1): 105–111. doi:10.1164/rccm.200409-1298oc. PMC 2662978. PMID 16210669.
- Avila, NA; Bechtle, J; Dwyer, AJ; Ferrans, VJ; Moss, J (2001). "Lymphangioleiomyomatosis: CT of diurnal variation of lymphangioleiomyomatosis". Radiology. 221 (2): 415–421. doi:10.1148/radiol.2212001448. PMID 11687685.
- Avila, NA; Dwyer, AJ; Rabel, A; Moss, J (2007). "Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: comparison of CT features". Radiology. 242 (1): 277–285. doi:10.1148/radiol.2421051767. PMC 2940246. PMID 17105849.
- Avila, NA; Kelly, JA; Chu, SC; Dwyer, AJ; Moss, J (2000). "Lymphangioleiomyomatosis: abdominopelvic CT and US findings". Radiology. 216 (1): 147–153. doi:10.1148/radiology.216.1.r00jl42147. PMID 10887241.
- Matsui, K; Tatsuguchi, A; Valencia, J; Yu, Z; Bechtle, J; Beasley, MB; Avila, NA; Travis, WD; Moss, J; Ferrans, VJ (2000). "Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases". Hum Pathol. 31 (10): 1242–1248. doi:10.1053/hupa.2000.18500. PMID 11070117.
- Ferrans, VJ; Yu, ZX; Nelson, WK; Valencia, JC; Tatsuguchi, A; Avila, NA; Riemenschn, W; Matsui, K; Travis, WD; Moss, J (2000). "Lymphangioleiomyomatosis (LAM) (A review of clinical and morphological features)". Journal of Nippon Medical School. 67 (1): 311–329. doi:10.1272/jnms.67.311. PMID 11031360.
- Taveira-DaSilva, AM; Steagall, WK; Moss, J (2006). "Lymphangioleiomyomatosis". Cancer Control. 13 (4): 276–285. doi:10.1177/107327480601300405. hdl:2042/44594. PMID 17075565.
- Crino, PB; Nathanson, KL; Henske, EP (2006). "The tuberous sclerosis complex". N Engl J Med. 355 (13): 1345–1356. doi:10.1056/nejmra055323. PMID 17005952. S2CID 3579356.
- McCormack FX, Travis WD, Colby TV, Henske EP, Moss J (December 2012). "Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm". Am. J. Respir. Crit. Care Med. 186 (12): 1210–2. doi:10.1164/rccm.201205-0848OE. PMC 3622443. PMID 23250499.
- van Slegtenhorst, M; de Hoogt, R; Hermans, C; Nellist, M; Janssen, B; Verhoef, S; Lindhout, D; van den Ouweland, A; Halley, D; Young, J; Burley, M; Jeremiah, S; Woodward, K; Nahmias, J; Fox, M; Ekong, R; Osborne, J; Wolfe, J; Povey, S; Snell, RG; Cheadle, JP; Jones, AC; Tachataki, M; Ravine, D; Sampson, JR; Reeve, MP; Richardson, P; Wilmer, F; Munro, C; Hawkins, TL; Sepp, T; Ali, JB; Ward, S; Green, AJ; Yates, JR; Kwiatkowska, J; Henske, EP; Short, MP; Haines, JH; Jozwiak, S; Kwiatkowski, DJ (1997). "Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34". Science. 277 (5327): 805–808. doi:10.1126/science.277.5327.805. PMID 9242607.
- Strizheva, GD; Carsillo, T; Kruger, WD; Sullivan, EJ; Ryu, JH; Henske, EP (2001). "The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis". Am J Respir Crit Care Med. 163 (1): 253–258. doi:10.1164/ajrccm.163.1.2005004. PMID 11208653.
- Carsillo, T; Astrinidis, A; Henske, EP (2000). "Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis". Proc Natl Acad Sci U S A. 97 (11): 6085–6090. Bibcode:2000PNAS...97.6085C. doi:10.1073/pnas.97.11.6085. PMC 18562. PMID 10823953.
- Badri, KR; Gao, L; Hyjek, E; Schuger, N; Schuger, L; Qin, W; Chekaluk, Y; Kwiatkowski, DJ; Zhe, X (2013). "Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis". Am J Respir Crit Care Med. 187 (6): 663–665. doi:10.1164/ajrccm.187.6.663. PMC 3733437. PMID 23504366.
- Knudson, AG, Jr (1971). "Mutation and cancer: statistical study of retinoblastoma". Proc Natl Acad Sci U S A. 68 (4): 820–823. Bibcode:1971PNAS...68..820K. doi:10.1073/pnas.68.4.820. PMC 389051. PMID 5279523.
- Knudson, AG (2001). "Two genetic hits (more or less) to cancer". Nat Rev Cancer. 1 (2): 157–162. doi:10.1038/35101031. PMID 11905807. S2CID 20201610.
- Henske, EP; Scheithauer, BW; Short, MP; Wollmann, R; Nahmias, J; Hornigold, N; van Slegtenhorst, M; Welsh, CT; Kwiatkowski, DJ (1996). "Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions". Am J Hum Genet. 59 (2): 400–406. PMC 1914733. PMID 8755927.
- Yu, J; Astrinidis, A; Henske, EP (2001). "Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis". Am J Respir Crit Care Med. 164 (8): 1537–1540. doi:10.1164/ajrccm.164.8.2104095. PMID 11704609.
- Smolarek, TA; Wessner, LL; McCormack, FX; Mylet, JC; Menon, AG; Henske, EP (1998). "Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis". Am J Hum Genet. 62 (4): 810–815. doi:10.1086/301804. PMC 1377043. PMID 9529362.
- Crooks, DM; Pacheco-Rodriguez, G; DeCastro, RM; McCoy, JP; Wang, JA; Kumaki, F; Darling, T; Moss, J (2004). "Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis". Proc Natl Acad Sci U S A. 101 (50): 17462–17467. Bibcode:2004PNAS..10117462C. doi:10.1073/pnas.0407971101. PMC 536045. PMID 15583138.
- Cai, X; Pacheco-Rodriguez, G; Fan, QY; Haughey, M; Samsel, L; El-Chemaly, S; Wu, HP; McCoy, JP; Steagall, WK; Lin, JP; Darling, TN; Moss, J (2010). "Phenotypic characterization of disseminated cells with TSC2 loss of heterozygosity in patients with lymphangioleiomyomatosis". Am J Respir Crit Care Med. 182 (11): 1410–1418. doi:10.1164/rccm.201003-0489oc. PMC 3029931. PMID 20639436.
- Karbowniczek, M; Astrinidis, A; Balsara, BR; Testa, JR; Lium, JH; Colby, TV; McCormack, FX; Henske, EP (2003). "Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism". Am J Respir Crit Care Med. 167 (7): 976–982. doi:10.1164/rccm.200208-969oc. PMID 12411287.
- Sato, T; Seyama, K; Fujii, H; Maruyama, H; Setoguchi, Y; Iwakami, S; Fukuchi, Y; Hino, O (2002). "Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis". J Hum Genet. 47 (1): 20–28. doi:10.1007/s10038-002-8651-8. PMID 11829138. S2CID 25627228.
- Dabora, SL; Jozwiak, S; Franz, DN; Roberts, PS; Nieto, A; Chung, J; Choy, YS; Reeve, MP; Thiele, E; Egelhoff, JC; Kasprzyk-Obara, J; Domanska-Pakiela, D; Kwiatkowski, DJ (2001). "Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs". Am J Hum Genet. 68 (1): 64–80. doi:10.1086/316951. PMC 1234935. PMID 11112665.
- Goncharova, EA; Goncharov, DA; Eszterhas, A; Hunter, DS; Glassberg, MK; Yeung, RS; Walker, CL; Noonan, D; Kwiatkowski, DJ; Chou, MM; Panettieri, RA Jr; Krymskaya, VP (2002). "Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM)". J Biol Chem. 277 (34): 30958–30967. doi:10.1074/jbc.m202678200. PMID 12045200. S2CID 41183449.
- Kwiatkowski, DJ; Zhang, H; Bandura, JL; Heiberger, KM; Glogauer, M; el-Hashemite, N; Onda, H (2002). "A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells". Hum Mol Genet. 11 (5): 525–534. doi:10.1093/hmg/11.5.525. PMID 11875047.
- McCormack, FX; Inoue, Y; Moss, J; Singer, LG; Strange, C; Nakata, K; Barker, AF; Chapman, JT; Brantly, ML; Stocks, JM; Brown, KK; Lynch, JP, 3rd; Goldberg, HI; Young, LR; Kinder, BW; Downey, GP; Sullivan, EJ; Colby, TV; McKay, RT; Cohen, MM; Korbee, L; Taveira-DaSilva, AM; Lee, HS; Krischer, JP; Trapnell, BC (2011). "Efficacy and safety of sirolimus in lymphangioleiomyomatosis". N Engl J Med. 364 (17): 1595–1606. doi:10.1056/nejmoa1100391. PMC 3118601. PMID 21410393.
- Bissler, JJ; McCormack, FX; Young, LR; Elwing, JM; Chuck, G; Leonard, JM; Schmithorst, VJ; Laor, T; Brody, AS; Bean, J; Salisbury, S; Franz, DN (2008). "Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis". N Engl J Med. 358 (2): 140–151. doi:10.1056/nejmoa063564. PMC 3398441. PMID 18184959.
- Guertin, DA; Sabatini, DM (2007). "Defining the role of mTOR in cancer". Cancer Cell. 12 (1): 9–22. doi:10.1016/j.ccr.2007.05.008. PMID 17613433.
- Hara, K; Maruki, Y; Long, X; Yoshino, K; Oshiro, N; Hidayat, S; Tokunaga, C; Avruch, J; Yonezawa, K (2002). "Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action". Cell. 110 (2): 177–189. doi:10.1016/s0092-8674(02)00833-4. PMID 12150926. S2CID 6438316.
- Kim, DH; Sarbassov, DD; Ali, SM; King, JE; Latek, RR; Erdjument-Bromage, H; Tempst, P; Sabatini, DM (2002). "mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery". Cell. 110 (2): 163–175. doi:10.1016/s0092-8674(02)00808-5. PMID 12150925. S2CID 4656930.
- Schalm, SS; Fingar, DC; Sabatini, DM; Blenis, J (2003). "TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function". Curr Biol. 13 (10): 797–806. doi:10.1016/s0960-9822(03)00329-4. PMID 12747827. S2CID 10326807.
- Frias, MA; Thoreen, CC; Jaffe, JD; Schroder, W; Sculley, T; Carr, SA; Sabatini, DM (2006). "mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s". Curr Biol. 16 (18): 1865–1870. doi:10.1016/j.cub.2006.08.001. PMID 16919458. S2CID 8239162.
- Jacinto, E; Facchinetti, V; Liu, D; Soto, N; Wei, S; Jung, SY; Huang, Q; Qin, J; Su, B (2006). "SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity". Cell. 127 (1): 125–137. doi:10.1016/j.cell.2006.08.033. PMID 16962653. S2CID 230319.
- Laplante, M; Sabatini, DM (2009). "mTOR signaling at a glance". J Cell Sci. 122 (Pt 20): 3589–3594. doi:10.1242/jcs.051011. PMC 2758797. PMID 19812304.
- Jacinto, E; Loewith, R; Schmidt, A; Lin, S; Rüegg, MA; Hall, A; Hall, MN (2004). "Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive". Nat Cell Biol. 6 (11): 1122–1128. doi:10.1038/ncb1183. PMID 15467718. S2CID 13831153.
- Sarbassov, DD; Ali, SM; Kim, DH; Guertin, DA; Latek, RR; Erdjument-Bromage, H; Tempst, P; Sabatini, DM (2004). "Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton". Curr Biol. 14 (14): 1296–1302. doi:10.1016/j.cub.2004.06.054. PMID 15268862. S2CID 4658268.
- Zoncu, R; Efeyan, A; Sabatini, DM (2011). "mTOR: from growth signal integration to cancer, diabetes and ageing". Nat Rev Mol Cell Biol. 12 (1): 21–35. doi:10.1038/nrm3025. PMC 3390257. PMID 21157483.
- Saci, A; Cantley, LC; Carpenter, CL (2011). "Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size". Mol Cell. 42 (1): 50–61. doi:10.1016/j.molcel.2011.03.017. PMC 3750737. PMID 21474067.
- Goncharova, E; Goncharov, D; Noonan, D; Krymskaya, VP (2004). "TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase". J Cell Biol. 167 (6): 1171–1182. doi:10.1083/jcb.200405130. PMC 2172598. PMID 15611338.
- Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP (April 2006). "Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis". Am. J. Respir. Cell Mol. Biol. 34 (4): 473–80. doi:10.1165/rcmb.2005-0374OC. PMC 2644208. PMID 16388022.
- Goncharova, EA; Goncharova, DA; Li, H; Pimtong, W; Lu, S; Khavin, I; Krymskaya, VP (2011). "mTORC2 is required for proliferation and survival of TSC2-null cells". Mol Cell Biol. 31 (12): 2484–2498. doi:10.1128/mcb.01061-10. PMC 3133430. PMID 21482669.
- El-Hashemite N, Kwiatkowski DJ (September 2005). "Interferon-gamma-JAK-STAT signaling in pulmonary lymphangioleiomyomatosis and renal angiomyolipoma: a potential therapeutic target". Am. J. Respir. Cell Mol. Biol. 33 (3): 227–30. doi:10.1165/rcmb.2005-0152RC. PMC 2715313. PMID 15994429.
- El-Hashemite, N; Zhang, H; Walker, V; Hoffmeister, KM; Kwiatkowski, DJ (2004). "Perturbed IFN-gamma-Jak-signal transducers and activators of transcription signaling in tuberous sclerosis mouse models: synergistic effects of rapamycin-IFN-gamma treatment". Cancer Res. 64 (10): 3436–3443. doi:10.1158/0008-5472.can-03-3609. PMID 15150095. S2CID 12194895.
- Goncharova EA, Goncharov DA, Chisolm A, Spaits MS, Lim PN, Cesarone G, Khavin I, Tliba O, Amrani Y, Panettieri RA, Krymskaya VP (March 2008). "Interferon beta augments tuberous sclerosis complex 2 (TSC2)-dependent inhibition of TSC2-null ELT3 and human lymphangioleiomyomatosis-derived cell proliferation". Mol. Pharmacol. 73 (3): 778–88. doi:10.1124/mol.107.040824. PMID 18094073. S2CID 19163380.
- Goncharova EA, Goncharov DA, Damera G, Tliba O, Amrani Y, Panettieri RA, Krymskaya VP (October 2009). "Signal transducer and activator of transcription 3 is required for abnormal proliferation and survival of TSC2-deficient cells: relevance to pulmonary lymphangioleiomyomatosis". Mol. Pharmacol. 76 (4): 766–77. doi:10.1124/mol.109.057042. PMC 2769052. PMID 19596836.
- Parkhitko, A; Myachina, F; Morrison, TA; Hindi, KM; Auricchio, N; Karbowniczek, M; Wu, JJ; Finkel, T; Kwiatkowski, DJ; Yu, JJ; Henske, EP (2011). "Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent". Proc Natl Acad Sci U S A. 108 (30): 12455–12460. Bibcode:2011PNAS..10812455P. doi:10.1073/pnas.1104361108. PMC 3145704. PMID 21746920.
- Henske, EP (2003). "Metastasis of benign tumor cells in tuberous sclerosis complex". Genes Chromosomes Cancer. 38 (4): 376–381. doi:10.1002/gcc.10252. PMID 14566858. S2CID 22211249.
- Henske EP, McCormack FX (November 2012). "Lymphangioleiomyomatosis - a wolf in sheep's clothing". J. Clin. Invest. 122 (11): 3807–16. doi:10.1172/JCI58709. PMC 3484429. PMID 23114603.
- Zhe X, Yang Y, Jakkaraju S, Schuger L (April 2003). "Tissue inhibitor of metalloproteinase-3 downregulation in lymphangioleiomyomatosis: potential consequence of abnormal serum response factor expression". Am. J. Respir. Cell Mol. Biol. 28 (4): 504–11. doi:10.1165/rcmb.2002-0124OC. PMID 12654640.
- Chang WY, Clements D, Johnson SR (September 2010). "Effect of doxycycline on proliferation, MMP production, and adhesion in LAM-related cells". Am. J. Physiol. Lung Cell Mol. Physiol. 299 (3): L393–400. doi:10.1152/ajplung.00437.2009. PMID 20581100. S2CID 23947917.
- Glassberg MK, Elliot SJ, Fritz J, Catanuto P, Potier M, Donahue R, Stetler-Stevenson W, Karl M (May 2008). "Activation of the estrogen receptor contributes to the progression of pulmonary lymphangioleiomyomatosis via matrix metalloproteinase-induced cell invasiveness". J. Clin. Endocrinol. Metab. 93 (5): 1625–33. doi:10.1210/jc.2007-1283. PMID 18285421.
- Lee PS, Tsang SW, Moses MA, Trayes-Gibson Z, Hsiao LL, Jensen R, Squillace R, Kwiatkowski DJ (February 2010). "Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells". Am. J. Respir. Cell Mol. Biol. 42 (2): 227–34. doi:10.1165/rcmb.2009-0050OC. PMC 2822984. PMID 19395678.
- Moir LM, Ng HY, Poniris MH, Santa T, Burgess JK, Oliver BG, Krymskaya VP, Black JL (September 2011). "Doxycycline inhibits matrix metalloproteinase-2 secretion from TSC2-null mouse embryonic fibroblasts and lymphangioleiomyomatosis cells". Br. J. Pharmacol. 164 (1): 83–92. doi:10.1111/j.1476-5381.2011.01344.x. PMC 3171862. PMID 21418186.
- Kumasaka, T; Seyama, K; Mitani, K; Souma, S; Kashiwagi, S; Hebisawa, A; Sato, T; Kubo, H; Gomi, K; Shibuya, K; Fukuchi, Y; Suda, K (2005). "Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis". Am J Surg Pathol. 29 (10): 1356–1366. doi:10.1097/01.pas.0000172192.25295.45. PMID 16160479. S2CID 35257926.
- Seyama K, Mitani K, Kumasaka T, Gupta SK, Oommen S, Liu G, Ryu JH, Vlahakis NE (April 2010). "Lymphangioleiomyoma cells and lymphatic endothelial cells: expression of VEGFR-3 in lymphangioleiomyoma cell clusters". Am. J. Pathol. 176 (4): 2051–2, author reply 2052–4. doi:10.2353/ajpath.2010.091239. PMC 2843492. PMID 20203284.
- Taveira-DaSilva, AM; Hathaway, O; Stylianou, M; Moss, J (2011). "Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus". Ann Intern Med. 154 (12): 797–805. doi:10.7326/0003-4819-154-12-201106210-00007. PMC 3176735. PMID 21690594.
- Glasgow CG, El-Chemaly S, Moss J (September 2012). "Lymphatics in lymphangioleiomyomatosis and idiopathic pulmonary fibrosis". Eur Respir Rev. 21 (125): 196–206. doi:10.1183/09059180.00009311. PMC 4241262. PMID 22941884.
- Glasgow CG, Taveira-DaSilva A, Pacheco-Rodriguez G, Steagall WK, Tsukada K, Cai X, El-Chemaly S, Moss J (December 2009). "Involvement of lymphatics in lymphangioleiomyomatosis". Lymphat Res Biol. 7 (4): 221–8. doi:10.1089/lrb.2009.0017. PMC 2883505. PMID 20143921.
- Glasgow CG, Taveira-Dasilva AM, Darling TN, Moss J (2008). "Lymphatic involvement in lymphangioleiomyomatosis". Ann. N. Y. Acad. Sci. 1131 (1): 206–14. Bibcode:2008NYASA1131..206G. doi:10.1196/annals.1413.018. PMC 3392168. PMID 18519973.
- Seyama, K; Kumasaka, T; Souma, S; Sato, T; Kurihara, M; Mitani, K; Tominaga, S; Fukuchi, Y (2006). "Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis". Lymphat Res Biol. 4 (3): 143–152. doi:10.1089/lrb.2006.4.143. PMID 17034294.
- Young, LR; Vandyke R, Gulleman, PM; Inoue, Y; Brown, KK; Schmidt, LS; Linehan, WM; Hajjar, F; Kinder, BW; Trapnell, BC; Bissler, JJ; Franz, DN; McCormack, FX (2010). "Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases". Chest. 138 (3): 674–681. doi:10.1378/chest.10-0573. PMC 2940071. PMID 20382711.
- Young L, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP, Goldberg HJ, Downey GP, Swigris JJ, Taveira-DaSilva AM, Krischer JP, Trapnell BC, McCormack FX (August 2013). "Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial". Lancet Respir Med. 1 (6): 445–52. doi:10.1016/S2213-2600(13)70090-0. PMC 3804556. PMID 24159565.
- Achen, MG; Jeltsch, M; Kukk, E; Mäkinen, T; Vitali, A; Wilks, AF; Alitalo, K; Stacker, SA (1998). "Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4)". Proc Natl Acad Sci U S A. 95 (2): 548–553. Bibcode:1998PNAS...95..548A. doi:10.1073/pnas.95.2.548. PMC 18457. PMID 9435229.
- Karnezis, T; Shayan, R; Caesar, C; Roufail, S; Harris, NC; Ardipradja, K; Zhang, YF; Williams, SP; Farnsworth, RH; Chai, MG; Rupasinghe, TW; Tull, DL; Baldwin, ME; Sloan, EK; Fox, SB; Achen, MG; Stacker, SA (2012). "VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium". Cancer Cell. 21 (2): 181–195. doi:10.1016/j.ccr.2011.12.026. PMID 22340592.
- Stacker, SA; Williams, SP; Karnezis, T; Shayan, R; Fox, SB; Achen, MG (2014). "Lymphangiogenesis and lymphatic vessel remodelling in cancer". Nat Rev Cancer. 14 (3): 159–172. doi:10.1038/nrc3677. PMID 24561443. S2CID 6976027.
- Achen, MG; Williams, RA; Baldwin, ME; Lai, P; Roufail, S; Alitalo, K; Stacker, SA (2002). "The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer". Growth Factors. 20 (2): 99–107. doi:10.1080/08977190290031969. PMID 12148568. S2CID 38782648.
- Davis JM, Hyjek E, Husain AN, Shen L, Jones J, Schuger LA (August 2013). "Lymphatic endothelial differentiation in pulmonary lymphangioleiomyomatosis cells". J. Histochem. Cytochem. 61 (8): 580–90. doi:10.1369/0022155413489311. PMC 3724387. PMID 23609227.
- Baldwin, ME; Catimel, B; Nice, EC; Roufail, S; Hall, NE; Stenvers, KL; Karkkainen, MJ; Alitalo, K; Stacker, SA; Achen, MG (2001). "The specificity of receptor binding by vascular endothelial growth factor-d is different in mouse and man". J Biol Chem. 276 (22): 19166–19171. doi:10.1074/jbc.m100097200. PMID 11279005. S2CID 41677159.
- Baldwin, ME; Halford, MM; Roufail, S; Williams, RA; Hibbs, ML; Grail, D; Kubo, H; Stacker, SA; Achen, MG (2005). "Vascular endothelial growth factor D is dispensable for development of the lymphatic system". Mol Cell Biol. 25 (6): 2441–2449. doi:10.1128/mcb.25.6.2441-2449.2005. PMC 1061605. PMID 15743836.
- Cudzilo, CJ; Szczesniak, RD; Brody, AS; Rattan, MS; Krueger, DA; Bissler, JJ; Franz, DN; McCormack, FX; Young, LR (2013). "Lymphangioleiomyomatosis screening in women with tuberous sclerosis". Chest. 144 (2): 578–585. doi:10.1378/chest.12-2813. PMID 23539171.
- Gupta, N; Meraj, R; Tanase, D; James, LE; Seyama, K; Lynch, DA; Akira, M; Meyer, CA; Ruoss, SJ; Burger, CD; Young, LR; Almoosa, KF; Veeraraghavan, S; Barker, AF; Lee, AS; Dilling, DF; Inoue, Y; Cudzilo, CJ; Zafar, MA; McCormack, FX (2015). "Accuracy of chest high-resolution computed tomography in diagnosing diffuse cystic lung diseases". Eur Respir J. 46 (4): 1196–1199. doi:10.1183/13993003.00570-2015. PMID 26160866. S2CID 12990858.
- Johnson, SR; Cordier, JF; Lazor, R; Cottin, V; Costabel, U; Harari, S; Reynaud-Gaubert, M; Boehler, A; Brauner, M; Popper, H; Bonetti, F; Kingswood, C (2010). "European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis". Eur Respir J. 35 (1): 14–26. doi:10.1183/09031936.00076209. PMID 20044458. S2CID 8775139.
- Ye, L; Jin, M; Bai, C (2010). "Clinical analysis of patients with pulmonary lymphangioleiomyomatosis (PLAM) in mainland China". Respir Med. 104 (10): 1521–1526. doi:10.1016/j.rmed.2010.05.003. PMID 20627505.
- Torre, O; Harari, S (2010). "The diagnosis of cystic lung diseases: a role for bronchoalveolar lavage and transbronchial biopsy?". Respir Med. 104 (Suppl 1): S81-5. doi:10.1016/j.rmed.2010.03.021. PMID 20430602.
- Mitani, K; Kumasaka, T; Takemura, H; Hayashi, T; Gunji, Y; Kunogi, M; Akiyoshi, T; Takahashi, K; Suda, K; Seyama, K (2009). "Cytologic, immunocytochemical and ultrastructural characterization of lymphangioleiomyomatosis cell clusters in chylous effusions of patients with lymphangioleiomyomatosis". Acta Cytol. 53 (4): 402–409. doi:10.1159/000325340. PMID 19697724. S2CID 3353292.
- Ohara, T; Oto, T; Miyoshi, K; Tao, H; Yamane, M; Toyooka, S; Okazaki, M; Date, H; Sano, Y (2008). "Sirolimus ameliorated post lung transplant chylothorax in lymphangioleiomyomatosis". Ann Thorac Surg. 86 (6): e7-8. doi:10.1016/j.athoracsur.2008.07.062. PMID 19021963.
- Yamauchi, M; Nakahara, H; Uyama, K; Tsujimoto, A; Tamai, M; Aozasa, K (2000). "Cytologic finding of chyloascites in lymphangioleiomyomatosis. A case report". Acta Cytol. 44 (6): 1081–1084. doi:10.1159/000328602. PMID 11127739. S2CID 3374041.
- Muller, NL; Chiles, C; Kullnig, P (1990). "Pulmonary lymphangiomyomatosis: Correlation of CT with radiographic and functional findings". Radiology. 175 (2): 335–339. doi:10.1148/radiology.175.2.2326457. PMID 2326457.
- Popper, HH; Juettner-Smolle, FM; Pongratz, MG (1991). "Micronodular hyperplasia of type II pneumocytes—A new lung lesion associated with tuberous sclerosis". Histopathology. 18 (4): 347–354. doi:10.1111/j.1365-2559.1991.tb00856.x. PMID 2071093. S2CID 29457399.
- Lantuejoul, S; Ferretti, G; Negoescu, A; Parent, B; Brambilla, E (1997). "Multifocal alveolar hyperplasia associated with lymphangioleiomyomatosis in tuberous sclerosis". Histopathology. 30 (6): 570–575. doi:10.1046/j.1365-2559.1997.4600811.x. PMID 9205862. S2CID 38522149.
- Muir, TE; Leslie, KO; Popper, H; Kitaichi, M; Gagné, E; Emelin, JK; Vinters, HV; Colby, TV (1998). "Micronodular pneumocyte hyperplasia". Am J Surg Pathol. 22 (4): 465–472. doi:10.1097/00000478-199804000-00012. PMID 9537475.
- Cancellieri, A; Poletti, V; Corrin, B (2002). "Respiratory failure due to micronodular type ii pneumocyte hyperplasia". Histopathology. 41 (3): 263–265. doi:10.1046/j.1365-2559.2002.01433.x. PMID 12207789. S2CID 7284904.
- Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX (June 2015). "Diffuse Cystic Lung Disease. Part I". Am. J. Respir. Crit. Care Med. 191 (12): 1354–66. doi:10.1164/rccm.201411-2094CI. PMC 5442966. PMID 25906089.
- Gupta, N; Vassallo, R; Wikenheiser-Brokamp, KA; McCormack, FX (2015). "Diffuse Cystic Lung Disease. Part II". Am J Respir Crit Care Med. 192 (1): 17–29. doi:10.1164/rccm.201411-2096ci. PMC 5447298. PMID 25906201.
- Jiang, X; Kenerson, H; Aicher, L; Miyaoka, R; Eary, J; Bissler, J; Yeung, RS (2008). "The tuberous sclerosis complex regulates trafficking of glucose transporters and glucose uptake". Am J Pathol. 172 (6): 1748–1756. doi:10.2353/ajpath.2008.070958. PMC 2408433. PMID 18511518.
- Young, LR; Franz, DN; Nagarkatte, P; Fletcher, CD; Wikenheiser-Brokamp, KA; Galsky, MD; Corbridge, TC; Lam, AP; Gelfand, MJ; McCormack, FX (2009). "Utility of [18F]2-fluoro-2-deoxyglucose-PET in sporadic and tuberous sclerosis-associated lymphangioleiomyomatosis". Chest. 136 (3): 926–933. doi:10.1378/chest.09-0336. PMC 3198490. PMID 19349386.
- Johnson, SR; Tattersfield, AE (1998). "Pregnancy in lymphangioleiomyomatosis". Am J Respir Crit Care Med. 157: A807.
- Moss, J; DeCastro, R; Patronas, NJ; DaSilva, A (2001). "Meningiomas in lymphangioleiomyomatosis". J Am Med Assoc. 286 (15): 1879–1881. doi:10.1001/jama.286.15.1879. PMID 11597290.
- Hayashida, M; Seyama, K; Inoue, Y; Fujimoto, K; Kubo, K (2007). "The epidemiology of lymphangioleiomyomatosis in Japan: A nationwide cross-sectional study of presenting features and prognostic factors". Respirology. 12 (4): 523–530. doi:10.1111/j.1440-1843.2007.01101.x. PMID 17587419. S2CID 10081145.
- Taveira-DaSilva, AM; Hedin, C; Stylianou, MP; Travis, WD; Matsui, K; Ferrans, VJ; Moss, J (2001). "Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis". Am J Respir Crit Care Med. 164 (6): 1072–1076. doi:10.1164/ajrccm.164.6.2102125. PMID 11587999.
- Yen, KT; Putzke, JD; Staats, BA; Burger, CD (2005). "The prevalence of acute response to bronchodilator in pulmonary lymphangioleiomyomatosis". Respirology. 10 (5): 643–648. doi:10.1111/j.1440-1843.2005.00762.x. PMID 16268919. S2CID 29523844.
- Burger, CD; Hyatt, RE; Staats, BA (1991). "Pulmonary mechanics in lymphangioleiomyomatosis". Am Rev Respir Dis. 143 (5 Pt 1): 1030–1033. doi:10.1164/ajrccm/143.5_Pt_1.1030. PMID 2024811.
- Taveira-DaSilva, AM; Stylianou, MP; Hedin, CJ; Kristof, AS; Avila, NA; Rabel, A; Travis, WD; Moss, J (2003). "Maximal oxygen uptake and severity of disease in lymphangioleiomyomatosis". Am J Respir Crit Care Med. 168 (12): 1427–1431. doi:10.1164/rccm.200206-593oc. PMID 12958050.
- Taveira-DaSilva, AM; Hathaway, OM; Sachdev, V; Shizukuda, Y; Birdsall, CW; Moss, J (2007). "Pulmonary artery pressure in lymphangioleiomyomatosis: An echocardiographic study". Chest. 132 (5): 1573–1578. doi:10.1378/chest.07-1205. PMC 2946895. PMID 17890459.
- Zafar, MA; McCormack, FX; Rahman, S; Tencza, C; Wikenheiser-Brokamp, KA; Young, LR; Shizukuda, Y; Elwing, JM (2013). "Pulmonary vascular shunts in exercise-intolerant patients with lymphangioleiomyomatosis". Am J Respir Crit Care Med. 188 (9): 1167–1170. doi:10.1164/rccm.201304-0618le. PMID 24180449.
- Corrin B, Liebow AA, Friedman PJ (May 1975). "Pulmonary lymphangiomyomatosis. A review". Am. J. Pathol. 79 (2): 348–82. PMC 1912658. PMID 1146965.
- Carrington CB, Cugell DW, Gaensler EA, Marks A, Redding RA, Schaaf JT, Tomasian A (December 1977). "Lymphangioleiomyomatosis. Physiologic-pathologic-radiologic correlations". Am. Rev. Respir. Dis. 116 (6): 977–95. doi:10.1164/arrd.1977.116.6.977 (inactive 2021-01-10). PMID 931190.CS1 maint: DOI inactive as of January 2021 (link)
- Matsumoto, Y; Horiba, K; Usuki, J; Chu, SC; Ferrans, VJ; Moss, J (1999). "Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis". Am J Respir Cell Mol Biol. 21 (3): 327–336. doi:10.1165/ajrcmb.21.3.3693. PMID 10460750.
- Hoon, V; Thung, SN; Kaneko, M; Unger, PD (1994). "HMB-45 reactivity in renal angiomyolipoma and lymphangioleiomyomatosis". Arch Pathol Lab Med. 118 (7): 732–734. PMID 8024410.
- McCarty, KS Jr; Mossler, JA; McLelland, R; Sieker, HO (1980). "Pulmonary lymphangiomyomatosis responsive to progesterone". N Engl J Med. 303 (25): 1461–1465. doi:10.1056/nejm198012183032506. PMID 7432404.
- Colley, MH; Geppert, E; Franklin, WA (1989). "Immunohistochemical detection of steroid receptors in a case of pulmonary lymphangioleiomyomatosis". Am J Surg Pathol. 13 (9): 803–807. doi:10.1097/00000478-198909000-00011. PMID 2764227.
- Gao, L; Yue, MM; Davis, J; Hyjek, E; Schuger, L (2014). "In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor". Virchows Arch. 464 (4): 495–503. doi:10.1007/s00428-014-1559-9. PMID 24570392. S2CID 8209801.
- Berger, U; Khaghani, A; Pomerance, A; Yacoub, MH; Coombes, RC (1990). "Pulmonary lymphangioleiomyomatosis and steroid receptors. An immunocytochemical study". Am J Clin Pathol. 93 (5): 609–614. doi:10.1093/ajcp/93.5.609. PMID 2183584.
- Logginidou, H; Ao, X; Henske, EP (2000). "Frequent estrogen and progesterone receptor immunoreactivity in renal angiomyolipomas from women with pulmonary lymphangioleiomyomatosis". Chest. 117 (1): 25–30. doi:10.1378/chest.117.1.25. PMID 10631194. S2CID 32090320.
- Henske EP, Ao X, Short MP, Greenberg R, Neumann HP, Kwiatkowski DJ, Russo I (July 1998). "Frequent progesterone receptor immunoreactivity in tuberous sclerosis-associated renal angiomyolipomas". Mod. Pathol. 11 (7): 665–8. PMID 9688188.
- Matsui K, K Riemenschneider W, Hilbert SL, Yu ZX, Takeda K, Travis WD, Moss J, Ferrans VJ (November 2000). "Hyperplasia of type II pneumocytes in pulmonary lymphangioleiomyomatosis". Arch. Pathol. Lab. Med. 124 (11): 1642–8. doi:10.1043/0003-9985(2000)124<1642:HOTIPI>2.0.CO;2 (inactive 2021-01-10). PMID 11079017.CS1 maint: DOI inactive as of January 2021 (link)
- Ussavarungsi, K; Hu, X; Scott, JP; Erasmus, DB; Mallea, JM; Alvarez, F; Lee, AS; Keller, CA; Ryu, JH; Burger, CD (2015). "Mayo clinic experience of lung transplantation in pulmonary lymphangioleiomyomatosis". Respir Med. 109 (10): 1354–1359. doi:10.1016/j.rmed.2015.08.014. PMID 26321137.
- Itkin, M (2014). "Lymphatic intervention is a new frontier of IR". J Vasc Interv Radiol. 25 (9): 1404–1405. doi:10.1016/j.jvir.2014.06.004. PMID 25150902.
- Ryu, JH; Doerr, CH; Fisher, SD; Sahn, SA (2003). "Chylothorax in lymphangioleiomyomatosis". Chest. 123 (2): 623–627. doi:10.1378/chest.123.2.623. PMID 12576391.
- De Luca, S; Terrone, C; Rossetti, SR (1999). "Management of renal angiomyolipoma: a report of 53 cases". BJU Int. 83 (3): 215–218. doi:10.1046/j.1464-410x.1999.00932.x. PMID 10233482. S2CID 24365886.
- Bissler, JJ; Kingswood, JC (2004). "Renal angiomyolipomata". Kidney Int. 66 (3): 924–934. doi:10.1111/j.1523-1755.2004.00838.x. PMID 15327383.
- Bissler, JJ; Kingswood, JC; Radzikowska, E; Zonnenberg, BA; Frost, M; Belousova, E; Sauter, M; Nonomura, N; Brakemeier, S; de Vries, PJ; Whittemore, VH; Chen, D; Sahmoud, T; Shah, G; Lincy, J; Lebwohl, D; Budde, K (2013). "Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial". Lancet. 381 (9869): 817–824. doi:10.1016/s0140-6736(12)61767-x. PMID 23312829. S2CID 25667463.
- Shen, A; Iseman, MD; Waldron, JA; King, TE (1987). "Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous estrogens". Chest. 91 (5): 782–785. doi:10.1378/chest.91.5.782. PMID 3032524.
- Goldberg, HJ; Harari, S; Cottin, V; Rosas, IO; Peters, E; Biswal, S; Cheng, Y; Khindri, S; Kovarik, JM; Ma, S; McCormack, FX; Henske, EP (2015). "Everolimus for the treatment of lymphangioleiomyomatosis: a phase II study". Eur Respir J. 46 (3): 783–794. doi:10.1183/09031936.00210714. PMID 26113676. S2CID 7779434.
- Chang, WY; Cane, JL; Kumaran, M; Lewis, S; Tattersfield, AE; Johnson, SR (2014). "A 2-year randomised placebo-controlled trial of doxycycline for lymphangioleiomyomatosis" (PDF). Eur Respir J. 43 (4): 1114–1123. doi:10.1183/09031936.00167413. PMID 24311763. S2CID 20074278.
- Oprescu, N; McCormack, FX; Byrnes, S; Kinder, BW (2013). "Clinical Predictors of Mortality and Cause of Death in Lymphangioleiomyomatosis: A Population-based Registry". Lung. 191 (1): 35–42. doi:10.1007/s00408-012-9419-3. PMID 23007140. S2CID 32978654.
- El-Hashemite, N; Walker, V; Zhang, H; Kwiatkowski, D (2003). "Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin". Cancer Res. 63 (17): 5173–5177. PMID 14500340.
- de la Fuente J, Páramo C, Román F, Pérez R, Masa C, de Letona JM (1993). "Lymphangioleiomyomatosis: unsuccessful treatment with luteinizing-hormone-releasing hormone analogues". Eur J Med. 2 (6): 377–8. PMID 8252188.
- Zhe, X; Yang, Y; Schuger, L (2005). "Imbalanced plasminogen system in lymphangioleiomyomatosis: potential role of serum response factor". Am J Respir Cell Mol Biol. 32 (1): 28–34. doi:10.1165/rcmb.2004-0289oc. PMID 15514113. S2CID 18536568.
- Rossi, GA; Balbi, B; Oddera, S; Lantero, S; Ravazzoni, C (1991). "Response to treatment with an analog of the luteinizing-hormone-releasing hormone in a patient with pulmonary lymphangioleiomyomatosis". Am Rev Respir Dis. 143 (1): 174–176. doi:10.1164/ajrccm/143.1.174. PMID 1824744.
- Schiavina M, Contini P, Fabiani A, Cinelli F, Di Scioscio V, Zompatori M, Campidelli C, Pileri SA (March 2007). "Efficacy of hormonal manipulation in lymphangioleiomyomatosis. A 20-year-experience in 36 patients". Sarcoidosis Vasc Diffuse Lung Dis. 24 (1): 39–50. doi:10.1007/s11083-007-9058-0. PMID 18069418. S2CID 44865113.
- Johnson, SR; Whale, CI; Hubbard, RB; Lewis, SA; Tattersfield, AE (2004). "Survival and disease progression in UK patients with lymphangioleiomyomatosis". Thorax. 59 (9): 800–803. doi:10.1136/thx.2004.023283. PMC 1747117. PMID 15333859.
- Aubry, MC; Myers, JL; Ryu, JH; Henske, EP; Logginidou, H; Jalal, SM; Tazelaar, HD (2000). "Pulmonary lymphangioleiomyomatosis in a man". Am J Respir Crit Care Med. 162 (2): 749–754. doi:10.1164/ajrccm.162.2.9911006. PMID 10934115.
- Schiavina, M; Di Scioscio, V; Contini, P; Cavazza, A; Fabiani, A; Barberis, M; Bini, A; Altimari, A; Cooke, RM; Grigioni, WF; D'Errico-Grigioni, A (2007). "Pulmonary lymphangioleiomyomatosis in a karyotypically normal man without tuberous sclerosis complex". Am J Respir Crit Care Med. 176 (1): 96–98. doi:10.1164/rccm.200610-1408cr. PMID 17431222.
- Harknett, EC; Chang, WY; Byrnes, S; Johnson, J; Lazor, R; Cohen, MM; Gray, B; Geiling, S; Telford, H; Tattersfield, AE; Hubbard, RB; Johnson, SR (2011). "Regional and national variability suggests underestimation of prevalence of lymphangioleiomyomatosis". Q J Med. 104 (11): 971–979. doi:10.1093/qjmed/hcr116. PMID 21764810.
- Adriaensen, ME; Schaefer-Prokop, CM; Duyndam, DA; Zonnenberg, BA; Prokop, M (2011). "Radiological evidence of lymphangioleiomyomatosis in female and male patients with tuberous sclerosis complex". Clin Radiol. 66 (7): 625–628. doi:10.1016/j.crad.2011.02.009. PMID 21459371.
- Wahedna, I; Cooper, S; Williams, J; Paterson, IC; Britton, JR; Tattersfield, AE (1994). "Relation of pulmonary lymphangioleiomyomatosis to use of the oral contraceptive pill and fertility in the UK: a national case control study". Thorax. 49 (9): 910–914. doi:10.1136/thx.49.9.910. PMC 475191. PMID 7940433.
- Oberstein, EM; Fleming, LE; Gómez-Marin, O; Glassberg, MK (2003). "Pulmonary lymphangioleiomyomatosis (LAM): examining oral contraceptive pills and the onset of disease". J Womens Health (Larchmt). 12 (1): 81–85. doi:10.1089/154099903321154176. PMID 12639372.
- Costello, LC; Hartman, TE; Ryu, JH (2000). "High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex". Mayo Clin Proc. 75 (6): 591–594. doi:10.4065/75.6.591. PMID 10852420.
- Moss, J; Avila, NA; Barnes, PM; Litzenberger, RA; Bechtle, J; Brooks, PG; Hedin, CJ; Hunsberger, S; Kristof, AS (2001). "Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex". Am J Respir Crit Care Med. 164 (4): 669–671. doi:10.1164/ajrccm.164.4.2101154. PMID 11520735.
- Muzykewicz, DA; Sharma, A; Muse, V; Numis, AL; Rajagopal, J; Thiele, EA (2009). "TSC1 and TSC2 mutations in patients with lymphangioleiomyomatosis and tuberous sclerosis complex". J Med Genet. 46 (7): 465–468. doi:10.1136/jmg.2008.065342. PMID 19419980. S2CID 22501227.
- Hughes E, Hodder RV (July 1987). "Pulmonary lymphangiomyomatosis complicating pregnancy. A case report". J Reprod Med. 32 (7): 553–7. PMID 3625622.
- Yockey, CC; Riepe, RE; Ryan, K (1986). "Pulmonary lymphangioleiomyomatosis complicated by pregnancy". Kans Med. 87 (10): 277–278, 293. PMID 3807098.
- Sleiman, C; Mal, H; Jebrak, G; Darne, C; Meeus, E; Dubois, F; Luisetti, M; Fournier, M; Pariente, R; Andreassian, B (1992). "Pulmonary lymphangiomyomatosis treated by single lung transplantation". Am Rev Respir Dis. 145 (4 Pt 1): 964–966. doi:10.1164/ajrccm/145.4_Pt_1.964. PMID 1554228.
- Kerr, LA; Blute, ML; Ryu, JH; Swensen, SJ; Malek, RS (1993). "Renal angiomyolipoma in association with pulmonary lymphangioleiomyomatosis: forme fruste of tuberous sclerosis?". Urology. 41 (5): 440–444. doi:10.1016/0090-4295(93)90504-4. PMID 8488612.
- Tazelaar, HD; Kerr, D; Yousem, SA; Saldana, MJ; Langston, C; Colby, TV (1993). "Diffuse pulmonary lymphangiomatosis". Hum Pathol. 24 (12): 1313–1322. doi:10.1016/0046-8177(93)90265-i. PMID 8276379.
- "About Us | About the LAM Foundation".
External links
| Classification | |
|---|---|
| External resources |