Neonatal withdrawal
Neonatal withdrawal or neonatal abstinence syndrome (NAS) is a withdrawal syndrome of infants after birth caused by in utero exposure to drugs of dependence.[1] There are two types of NAS: prenatal and postnatal. Prenatal NAS is caused by discontinuation of drugs taken by the pregnant mother, while postnatal NAS is caused by discontinuation of drugs directly to the infant.[2][3]
| Neonatal withdrawal | |
|---|---|
| Other names | Neonatal abstinence syndrome |
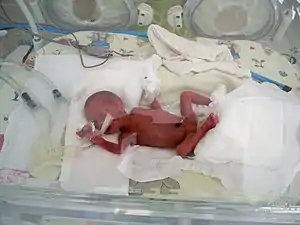 | |
| Prematurity can accompany withdrawal | |
| Specialty | Pediatrics |
Signs and symptoms
Drug and alcohol use during pregnancy can lead to many health problems in the fetus and baby, Neonatal Abstinence Syndrome(NAS). These issues may include:[4]
- Tremors (trembling)
- Irritability (excessive mood crying)
- Sleep problems
- High-pitched crying
- Tight muscle tone
- Hyperactive reflexes
- Seizures
- Yawning, stuffy nose, and sneezing
- Poor feeding and sucking reflex
- Vomiting
- Diarrhea
- Dehydration
- Sweating [5]
Causes
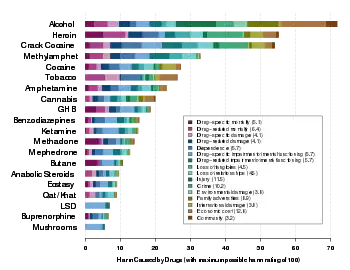
The drugs involved may be, for example, opioids, selective serotonin reuptake inhibitors (SSRIs), ethanol and benzodiazepines.[1] Some drugs may be more likely to cause the syndrome than others. Exposure to heroin and methadone claimed to be correlated with a 60% to 80% occurrence of neonatal withdrawal, whereas buprenorphine has been associated with a lower risk.[6] Studies however, demonstrate conflicting results.[7] Neonatal abstinence syndrome does not happen in prenatal cocaine exposure. Prematurity and exposure to other drugs may instead be the cause of symptoms.[8]
Mechanisms
Drugs and chemicals pass through the placenta that connects the baby to its mother in the womb. The baby becomes dependent on the drug along with the mother. If the mother continues to use the drugs within the week or so before delivery, the baby will be dependent on the drug at birth. Because the baby is no longer getting the drug after birth, withdrawal symptoms may occur as the drug is slowly cleared from the baby's system.[1] Nicotine, medications and alcohol have side effects related to unsafe higher dosages, but neonates may respond differently. Newborns are less able to metabolize drugs and therefore the substance stays in their system for a relatively longer length of time when compared to those who are older and have fully functioning livers and kidneys.[9]
Diagnosis
The presence of withdrawal in the neonate can be confirmed via a detailed medical history from the mother. In some cases neonatal drug withdrawal can be mistaken for central nervous system disorders.[10] Typically the tests that are ordered are CBC, hair analysis, drug screen (of mother and infant), thyroid levels, electrolytes, and blood glucose. Chest x-rays can confirm or infirm the presence of heart defects.[11][1] The diagnosis for babies with signs of withdrawal may be confirmed with drug tests of the baby's urine or stool. The mother's urine will also be tested.[1]
There are at least two different scoring systems for neonatal withdrawal syndrome. One difficulty with both is that they were developed to assess opiate withdrawal. The Finnegan scoring system is more widely used.[10]
Prevention
Neonatal withdrawal is prevented by the mother abstaining from substance abuse. In some cases, a prescribed medication may have to be discontinued during the pregnancy to prevent addiction by the baby. Early pre-natal care can identify addictive behaviors in the mother and family system. Referrals to treatment centers is appropriate.[11] Some prescribed medicines should not be stopped without medical supervision, or harm may result. Women can discuss all medicines, and alcohol and tobacco use with their health care provider and get assistance to help stop drug use as soon as possible. Indications that a woman needs help if she is:
- Using drugs non-medically
- Using drugs not prescribed to her
- Using alcohol or tobacco[1]
Treatment
Treatment depends on the drug involved, the infant's overall health, abstinence scores and whether the baby was born full-term or premature. First line treatment consists of supportive measures, such as providing a quiet environment, breastfeeding, and swaddling or holding the infant.[12] Clinicians will watch the newborn carefully for up to a week after birth for signs of withdrawal, feeding problems, and weight gain. Babies who vomit or who are very dehydrated may temporarily need intravenous fluids or feeding through a nasogastric tube.
Some babies with severe symptoms need medicines such as methadone and morphine to treat withdrawal symptoms. Buprenorphine may also be effective.[13]
These babies may need to stay in the hospital for weeks or months after birth. The goal of treatment is to prescribe the infant a drug similar to the one the mother used during pregnancy and slowly decrease the dose over time.[14] This helps wean the baby off the drug and relieves some withdrawal symptoms.
If the symptoms are severe, especially if other drugs were used, a second medicine such as phenobarbital or clonidine may be added. Breastfeeding may also be helpful if the mother is in a methadone or buprenorphine treatment program without other drug use.
Babies with this condition often have severe diaper rash or other areas of skin breakdown. This requires treatment with special ointment or cream. Babies may also have problems with feeding or slow growth. These problems may require higher-calorie feedings that provide greater nutrition and smaller portions given more often.[1] Objectives of management are to minimize negative outcomes and promote normal development.[15]
Supportive
Initial treatment should begin with non-pharmacological interventions in order to support maturation of the neonate. Techniques such as adjusting the physical environment by darkening the room and eliminating surrounding sounds work to lessen the neonate's visual and auditory stimuli.[16]
Non-medication based approaches to treat neonatal symptoms include swaddling the infant in a blanket, minimizing environmental stimuli, and monitoring sleeping and feeding patterns.[1] Breastfeeding promotes infant attachment and bonding and is associated with a decreased need for medication. These approaches may lessen the severity of NAS and lead to shorter hospital stays.[17]
Medication
Although non-pharmacological intervention remains first line treatment, pharmacological intervention when appropriate and indicated, can improve signs of neonatal withdrawal.[16]
Medication is used to relieve fever, seizures, and weight loss or dehydration.[15] When medication use for opiate withdrawal in newborn babies is deemed necessary, opiates are the treatment of choice; they are slowly tapered down to wean the baby off opiates.[18] Phenobarbital is sometimes used as an alternative but is less effective in suppressing seizures; however, phenobarbital is superior to diazepam for neonatal opiate withdrawal symptoms. In the case of sedative-hypnotic neonatal withdrawal, phenobarbital is the treatment of choice.[19][20] Clonidine is an emerging add-on therapy.[21]
Opioids such as neonatal morphine solution and methadone are commonly used to treat clinical symptoms of opiate withdrawal, but may prolong neonatal drug exposure and duration of hospitalization.[22] A study demonstrated a shorter wean duration in infants treated with methadone compared to those treated with diluted tincture of opium. When compared to morphine, methadone has a longer half-life in children, which allows for less frequent dosing intervals and steady serum concentrations to prevent neonatal withdrawal symptoms.[23]
Epidemiology
A 2012 study from the University of Michigan and the University of Pittsburgh published in the Journal of the American Medical Association analyzed information on 7.4 million discharges from 4,121 hospitals in 44 states, to measure trends and costs associated with NAS over the past decade. The study indicated that between 2000 and 2009, the number of mothers using opiates increased from 1.19 to 5.63 per 1,000 hospital births per year. Newborns with NAS were 19% more likely than all other hospital births to have low birthweight and 30% more likely to have respiratory complications. Between 2000 and 2009, total hospital charges for NAS cases, adjusted for inflation, are estimated to have increased from $190 million to $720 million.[24]
Neonatal abstinence syndrome in Canada are significant.[25][26]
References
- "Neonatal abstinence Syndrome". MedlinePlus. US Library of Medicine. 5 July 2017. Retrieved 27 July 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Neonatal Abstinence Syndrome on eMedicine
- Hall RW, Boyle E, Young T (October 2007). "Do ventilated neonates require pain management?". Seminars in Perinatology. 31 (5): 289–97. doi:10.1053/j.semperi.2007.07.002. PMID 17905183.
- "Neonatal abstinence syndrome: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 2020-07-27.
- "Default - Stanford Children's Health".
- Siu A, Robinson CA (July 2014). "Neonatal abstinence syndrome: essentials for the practitioner". The Journal of Pediatric Pharmacology and Therapeutics. 19 (3): 147–55. doi:10.5863/1551-6776-19.3.147. PMC 4187528. PMID 25309144.
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. (December 2010). "Neonatal abstinence syndrome after methadone or buprenorphine exposure". The New England Journal of Medicine. 363 (24): 2320–31. doi:10.1056/NEJMoa1005359. PMC 3073631. PMID 21142534.
- Mercer J (2009). "Claim 9: "Crack babies" can't be cured and will always have serious problems". Child Development: Myths and Misunderstandings. Thousand Oaks, Calif: Sage Publications, Inc. pp. 62–64. ISBN 978-1-4129-5646-8.
- Hamdan A (December 20, 2017). "Neonatal Abstinence Syndrome". Medscape. Archived from the original on September 14, 2017. Retrieved July 25, 2018.
- "Neonatal Abstinence Syndrome Clinical Presentations". Medscape. 27 November 2016. Retrieved 28 July 2017.
- Henry, p. 184.
- Wachman, Elisha M.; Houghton, Mary; Melvin, Patrice; Isley, Breanna C.; Murzycki, Jennifer; Singh, Rachana; Minear, Susan; MacMillan, Kathryn Dee L.; Banville, Debra; Walker, Amy; Mitchell, Teresa (October 2020). "A quality improvement initiative to implement the eat, sleep, console neonatal opioid withdrawal syndrome care tool in Massachusetts' PNQIN collaborative". Journal of Perinatology. 40 (10): 1560–1569. doi:10.1038/s41372-020-0733-y. ISSN 1476-5543. PMID 32678314. S2CID 220576891.
- Disher T, Gullickson C, Singh B, Cameron C, Boulos L, Beaubien L, Campbell-Yeo M (March 2019). "Pharmacological Treatments for Neonatal Abstinence Syndrome: A Systematic Review and Network Meta-analysis". JAMA Pediatrics. 173 (3): 234–243. doi:10.1001/jamapediatrics.2018.5044. PMC 6439896. PMID 30667476.
- Kraft WK, Stover MW, Davis JM (April 2016). "Neonatal abstinence syndrome: Pharmacologic strategies for the mother and infant". Seminars in Perinatology. 40 (3): 203–12. doi:10.1053/j.semperi.2015.12.007. PMC 4808371. PMID 26791055.
- McQueen K, Murphy-Oikonen J (December 2016). "Neonatal Abstinence Syndrome". The New England Journal of Medicine. 375 (25): 2468–2479. doi:10.1056/NEJMra1600879. PMID 28002715.
- Anbalagan S, Mendez MD (2020). Neonatal Abstinence Syndrome. StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31855342. Retrieved 2020-07-28.
- Pritham UA, Paul JA, Hayes MJ (March 2012). "Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome". Journal of Obstetric, Gynecologic, and Neonatal Nursing. 41 (2): 180–190. doi:10.1111/j.1552-6909.2011.01330.x. PMC 3407283. PMID 22375882.
- Hudak ML, Tan RC (February 2012). "Neonatal drug withdrawal". Pediatrics. 129 (2): e540-60. doi:10.1542/peds.2011-3212. PMID 22291123.
- Osborn DA, Jeffery HE, Cole MJ (October 2010). Osborn DA (ed.). "Opiate treatment for opiate withdrawal in newborn infants". The Cochrane Database of Systematic Reviews (10): CD002059. doi:10.1002/14651858.CD002059.pub3. PMID 20927730.
- Osborn DA, Jeffery HE, Cole MJ (October 2010). Osborn DA (ed.). "Sedatives for opiate withdrawal in newborn infants". The Cochrane Database of Systematic Reviews (10): CD002053. doi:10.1002/14651858.CD002053.pub3. PMID 20927729.
- Kraft WK, van den Anker JN (October 2012). "Pharmacologic management of the opioid neonatal abstinence syndrome". Pediatric Clinics of North America. 59 (5): 1147–65. doi:10.1016/j.pcl.2012.07.006. PMC 4709246. PMID 23036249.
- Logan BA, Brown MS, Hayes MJ (March 2013). "Neonatal abstinence syndrome: treatment and pediatric outcomes". Clinical Obstetrics and Gynecology. 56 (1): 186–92. doi:10.1097/GRF.0b013e31827feea4. PMC 3589586. PMID 23314720.
- Johnson MR, Nash DR, Laird MR, Kiley RC, Martinez MA (July 2014). "Development and implementation of a pharmacist-managed, neonatal and pediatric, opioid-weaning protocol". The Journal of Pediatric Pharmacology and Therapeutics. 19 (3): 165–73. doi:10.5863/1551-6776-19.3.165. PMC 4187529. PMID 25309146.
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM (May 2012). "Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009". JAMA. 307 (18): 1934–40. doi:10.1001/jama.2012.3951. PMID 22546608.
- Dow O (2012). "Neonatal Abstinence syndrome clinical practice guidelines for Ontatio" (PDF). Journal of Population Therapeutics and Clinical Pharmacology. 19: 488–506.
- Leslie K (2015). "Officials can't explain increase in North Bay babies born to addicted moms". CTV News.
Bibliography
- Henry N (2016). RN Maternal Newborn Nursing. Stilwell, KS: Assessment Technologies Institute. ISBN 9781565335691.
External links
| Wikiquote has quotations related to: Neonatal withdrawal |
| Classification | |
|---|---|
| External resources |