Nicotine dependence
Nicotine dependence[notes 1] is a state of dependence upon nicotine.[1] Nicotine dependence is a chronic, relapsing disease defined as a compulsive craving to use the drug, despite harmful social consequences.[5] Tolerance is another component of drug dependence.[6] Nicotine dependence develops over time as a person continues to use nicotine.[6] Nicotine dependence is a serious public health concern due to it being one of the leading causes of avoidable deaths worldwide. [7]
| Nicotine dependence | |
|---|---|
| Other names | tobacco dependence; tobacco use disorder |
| Video explanation | |
There are different ways of measuring nicotine dependence.[3] The five common dependence assessment scales are the Fagerström Test for Nicotine Dependence, the Diagnostic and Statistical Manual of Mental Disorders, the Cigarette Dependence Scale, the Nicotine Dependence Syndrome Scale, and the Wisconsin Inventory of Smoking Dependence Motives.[3] The long use of Fagerström Test for Nicotine Dependence is supported by the existence of significant preexisting research, and its conciseness.[3]
There are approximately 976 million smokers in the world.[8] There is an increased frequency of nicotine dependence in people with anxiety disorders.[9] Nicotine is a sympathomimetic stimulant[10] that attaches to nicotinic acetylcholine receptors in the brain.[11] Neuroplasticity within the brain's reward system occurs as a result of long-term nicotine use, leading to nicotine dependence.[1] There are genetic risk factors for developing dependence.[12] For instance, genetic markers for a specific type of nicotinic receptor (the α5-α3-β4 nicotine receptors) have been linked to increased risk for dependence.[12] Evidence-based medicine can double or triple a smoker's chances of quitting successfully.[13]
Definition
Nicotine dependence is defined as a neurobiological adaptation to repeated drug exposure that is manifested behaviorally by highly controlled or compulsive use; psychoactive effects such as tolerance, physical dependence, and pleasant effect; and nicotine-reinforced behavior, including an inability to quit despite harmful effects, a desire to quit, and repeated cessation attempts.[15] Nicotine dependence is a chronic, relapsing disease defined as a compulsive craving to use the drug, despite harmful social consequences; inability to control drug use; and onset of withdrawal-like symptoms when the drug is discontinued.[5] A 1988 Surgeon General report states, "Tolerance" is another aspect of drug addiction [dependence] whereby a given dose of a drug produces less effect or increasing doses are required to achieve a specified intensity of response. Physical dependence on the drug can also occur, and is characterized by a withdrawal syndrome that usually accompanies drug abstinence. After cessation of drug use, there is a strong tendency to relapse."[6]
Nicotine dependence leads to heavy smoking and causes severe withdrawal symptoms and relapse back to smoking.[6] Nicotine dependence develops over time as a person continues to use nicotine.[6] Teenagers do not have to be daily or long-term smokers to show withdrawal symptoms.[16] Relapse should not frustrate the nicotine user from trying to quit again.[13] A 2015 review found "Avoiding withdrawal symptoms is one of the causes of continued smoking or relapses during attempts at cessation, and the severity and duration of nicotine withdrawal symptoms predict relapse."[17] Symptoms of nicotine dependence include irritability, anger, impatience, and problems in concentrating.[18]
Diagnosis
There are different ways of measuring nicotine dependence.[3] The five common dependence assessment scales are the Fagerström Test for Nicotine Dependence, the Diagnostic and Statistical Manual of Mental Disorders, the Cigarette Dependence Scale, the Nicotine Dependence Syndrome Scale, and the Wisconsin Inventory of Smoking Dependence Motives.[3]
The Fagerström Test for Nicotine Dependence focuses on measuring physical dependence which is defined "as a state produced by chronic drug administration, which is revealed by the occurrence of signs of physiological dysfunction when the drug is withdrawn; further, this dysfunction can be reversed by the administration of drug".[3] The long use of Fagerström Test for Nicotine Dependence is supported by the existence of significant preexisting research, and its conciseness.[3]
The 4th edition of the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) had a nicotine dependence diagnosis which was defines as "...a cluster of cognitive, behavioral, and physiological symptoms..."[3] In the updated DSM-5 there is no nicotine dependence diagnosis, but rather Tobacco Use Disorder, which is defined as, "A problematic pattern of tobacco use leading to clinically significant impairment or distress, as manifested by at least 2 of the following [11 symptoms], occurring within a 12-month period."[19]
The Cigarette Dependence Scale was developed "to index dependence outcomes and not dependence mechanisms".[3] The Nicotine Dependence Syndrome Scale, "a 19-item self-report measure, was developed as a multidimensional scale to assess nicotine dependence".[3] The Wisconsin Inventory of Smoking Dependence Motives "is a 68-item measure developed to assess dependence as a motivational state".[3]
Mechanisms
Traditional cigarettes are the most common delivery device for nicotine. However, electronic cigarettes are becoming more popular.[20] Nicotine can also be delivered via other tobacco products such as chewing tobacco, snus, pipe tobacco, hookah, all of which can produce nicotine dependence.
Biomolecular
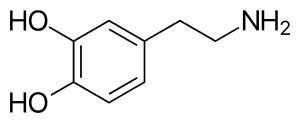
Pre-existing cognitive and mood disorders may influence the development and maintenance of nicotine dependence.[21] Nicotine is a parasympathomimetic stimulant[10] that binds to and activates nicotinic acetylcholine receptors in the brain,[11] which subsequently causes the release of dopamine and other neurotransmitters, such as norepinephrine, acetylcholine, serotonin, gamma-aminobutyric acid, glutamate, endorphins,[22] and several neuropeptides.[23] Repeated exposure to nicotine can cause an increase in the number of nicotinic receptors, which is believed to be a result of receptor desensitization and subsequent receptor upregulation.[22] This upregulation or increase in the number of nicotinic receptors significantly alters the functioning of the brain reward system.[24] With constant use of nicotine, tolerance occurs at least partially as a result of the development of new nicotinic acetylcholine receptors in the brain.[22] After several months of nicotine abstinence, the number of receptors go back to normal.[11] Nicotine also stimulates nicotinic acetylcholine receptors in the adrenal medulla, resulting in increased levels of adrenaline and beta-endorphin.[22] Its physiological effects stem from the stimulation of nicotinic acetylcholine receptors, which are located throughout the central and peripheral nervous systems.[25] Chronic nicotinic acetylcholine receptor activation from repeated nicotine exposure can induce strong effects on the brain, including changes in the brain's physiology, that result from the stimulation of regions of the brain associated with reward, pleasure, and anxiety.[26] These complex effects of nicotine on the brain are still not well understood.[26]
When these receptors are not occupied by nicotine, they are believed to produce withdrawal symptoms.[27] These symptoms can include cravings for nicotine, anger, irritability, anxiety, depression, impatience, trouble sleeping, restlessness, hunger, weight gain, and difficulty concentrating.[28]
Neuroplasticity within the brain's reward system occurs as a result of long-term nicotine use, leading to nicotine dependence.[1] There are genetic risk factors for developing dependence.[12] For instance, genetic markers for a specific type of nicotinic receptor (the α5-α3-β4 nicotine receptors) have been linked to increased risk for dependence.[12][29] The most well-known hereditary influence related to nicotine dependence is a mutation at rs16969968 in the nicotinic acetylcholine receptor CHRNA5, resulting in an amino acid alteration from aspartic acid to asparagine.[30] The single-nucleotide polymorphisms (SNPs) rs6474413 and rs10958726 in CHRNB3 are highly correlated with nicotine dependence.[31] Many other known variants within the CHRNB3–CHRNA6 nicotinic acetylcholine receptors are also correlated with nicotine dependence in certain ethnic groups.[31] There is a relationship between CHRNA5-CHRNA3-CHRNB4 nicotinic acetylcholine receptors and complete smoking cessation.[32] Increasing evidence indicates that the genetic variant CHRNA5 predicts the response to smoking cessation medicine.[32]
Psychosocial
In addition to the specific neurological changes in nicotinic receptors, there are other changes that occur as dependence develops. Through various conditioning mechanisms (operant and cue/classical), smoking comes to be associated with different mood and cognitive states as well as external contexts and cues.[24]
Treatment
There are treatments for nicotine dependence, although the majority of the evidence focuses on treatments for cigarette smokers rather than people who use other forms of tobacco (e.g., chew, snus, pipes, hookah, e-cigarettes). Evidence-based medicine can double or triple a smoker's chances of quitting successfully.[13]
Medication
There are eight major evidence-based medications for treating nicotine dependence: bupropion, cytisine (not approved for use in some countries, including the US), nicotine gum, nicotine inhaler, nicotine lozenge/mini-lozenge, nicotine nasal spray, nicotine patch, and varenicline.[33] These medications have been shown to significantly improve long-term (i.e., 6-months post-quit day) abstinence rates, especially when used in combination with psychosocial treatment.[13] The nicotine replacement treatments (i.e., patch, lozenge, gum) are dosed based on how dependent a smoker is—people who smoke more cigarettes or who smoke earlier in the morning use higher doses of nicotine replacement treatments. There is no consensus for remedies for tobacco use disorder among pregnant smokers who also use alcohol and stimulants.[4]
Psychosocial
Psychosocial interventions delivered in-person (individually or in a group) or over the phone (including mobile phone interventions) have been shown to effectively treat nicotine dependence.[33] These interventions focus on providing support for quitting and helping with smokers with problem-solving and developing healthy responses for coping with cravings, negative moods, and other situations that typically lead to relapse. The combination of pharmacotherapy and psychosocial interventions has been shown to be especially effective.[13]
Epidemiology
First-time nicotine users develop a dependence about 32% of the time.[34] There are approximately 976 million smokers in the world.[8] Estimates are that half of smokers (and one-third of former smokers) are dependent based on DSM criteria, regardless of age, gender or country of origin, but this could be higher if different definitions of dependence were used.[35] Recent data suggest that, in the United States, the rates of daily smoking and the number of cigarettes smoked per day are declining, suggesting a reduction in population-wide dependence among current smokers.[36] However, there are different groups of people who are more likely to smoke than the average population, such as those with low education or low socio-economic status and those with mental illness.[36] There is also evidence that among smokers, some subgroups may be more dependent than other groups. Men smoke at higher rates than do women and score higher on dependence indices; however, women may be less likely to be successful in quitting, suggesting that women may be more dependent by that criterion.[36][37] There is an increased frequency of nicotine dependence in people with anxiety disorders.[9] 6% of smokers who want to quit smoking each year are successful at quitting.[7] Nicotine withdrawal is the main factor hindering smoking cessation.[38] A 2010 World Health Organization report states, "Greater nicotine dependence has been shown to be associated with lower motivation to quit, difficulty in trying to quit, and failure to quit, as well as with smoking the first cigarette earlier in the day and smoking more cigarettes per day."[39] E-cigarettes may result in starting nicotine dependence again.[40] Greater nicotine dependence may result from dual use of traditional cigarettes and e-cigarettes.[40] Like tobacco companies did in the last century, there is a possibility that e-cigarettes could result in a new form of dependency on nicotine across the world.[41]
Concerns
Nicotine dependence results in substantial mortality, morbidity, and socio-economic impacts.[7] Nicotine dependence is a serious public health concern due to it being one of the leading causes of avoidable deaths worldwide.[7] The medical community is concerned that e-cigarettes may escalate global nicotine dependence, particularly among adolescents who are attracted to many of the flavored e-cigarettes.[42] There is strong evidence that vaping induces symptoms of dependence in users.[43] Many organizations such the World Health Organization, American Lung Association, and Australian Medical Association do not approve of vaping for quitting smoking in youth, making reference to concerns about their safety and the potential that experimenting with vaping may result in nicotine dependence and later tobacco use.[44]
Notes
See also
Bibliography
- Stratton, Kathleen; Kwan, Leslie Y.; Eaton, David L. (January 2018). Public Health Consequences of E-Cigarettes (PDF). National Academies of Sciences, Engineering, and Medicine. National Academies Press. pp. 1–774. doi:10.17226/24952. ISBN 978-0-309-46834-3. PMID 29894118.
References
- D'Souza MS, Markou A (2011). "Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments". Addict Sci Clin Pract. 6 (1): 4–16. PMC 3188825. PMID 22003417.CS1 maint: uses authors parameter (link)
- Stratton 2018, p. Dependence and Abuse Liability, 256.
- Piper, Megan; McCarthy, Danielle; Baker, Timothy (2006). "Assessing tobacco dependence: A guide to measure evaluation and selection". Nicotine & Tobacco Research. 8 (3): 339–351. doi:10.1080/14622200600672765. ISSN 1462-2203. PMID 16801292.
- Akerman, Sarah C.; Brunette, Mary F.; Green, Alan I.; Goodman, Daisy J.; Blunt, Heather B.; Heil, Sarah H. (2015). "Treating Tobacco Use Disorder in Pregnant Women in Medication-Assisted Treatment for an Opioid Use Disorder: A Systematic Review". Journal of Substance Abuse Treatment. 52: 40–47. doi:10.1016/j.jsat.2014.12.002. ISSN 0740-5472. PMC 4382443. PMID 25592332.
- Falcone, Mary; Lee, Bridgin; Lerman, Caryn; Blendy, Julie A. (2015). "Translational Research on Nicotine Dependence". Translational Neuropsychopharmacology. Current Topics in Behavioral Neurosciences. 28. pp. 121–150. doi:10.1007/7854_2015_5005. ISBN 978-3-319-33911-5. ISSN 1866-3370. PMC 3579204. PMID 26873019.
- U.S. Department of Health and Human Services (1988). The health consequences of smoking: Nicotine addiction: A report of the Surgeon General (PDF). U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health. DHHS Publication No. (CDC) 88-8406.
- Rachid, Fady (2016). "Neurostimulation techniques in the treatment of nicotine dependence: A review". The American Journal on Addictions. 25 (6): 436–451. doi:10.1111/ajad.12405. ISSN 1055-0496. PMID 27442267.
- Ng, M; Freeman, MK; Fleming, TD; Robinson, M; Dwyer-Lindgren, L; Thomson, B; Wollum, A; Sanman, E; Wulf, S; Lopez, AD; Murray, CJ; Gakidou, E (8 January 2014). "Smoking prevalence and cigarette consumption in 187 countries, 1980-2012". JAMA. 311 (2): 183–92. doi:10.1001/jama.2013.284692. PMID 24399557.
- Moylan, Steven; Jacka, Felice N; Pasco, Julie A; Berk, Michael (2012). "Cigarette smoking, nicotine dependence and anxiety disorders: a systematic review of population-based, epidemiological studies". BMC Medicine. 10 (1): 123. doi:10.1186/1741-7015-10-123. ISSN 1741-7015. PMC 3523047. PMID 23083451.
- Richard Beebe; Jeff Myers (19 July 2012). Professional Paramedic, Volume I: Foundations of Paramedic Care. Cengage Learning. pp. 640–. ISBN 978-1-133-71465-1.
- Bullen, Christopher (2014). "Electronic Cigarettes for Smoking Cessation". Current Cardiology Reports. 16 (11): 538. doi:10.1007/s11886-014-0538-8. ISSN 1523-3782. PMID 25303892.
- Saccone, NL; Culverhouse, RC; Schwantes-An, TH; Cannon, DS; Chen, X; Cichon, S; Giegling, I; Han, S; Han, Y; Keskitalo-Vuokko, K; Kong, X; Landi, MT; Ma, JZ; Short, SE; Stephens, SH; Stevens, VL; Sun, L; Wang, Y; Wenzlaff, AS; Aggen, SH; Breslau, N; Broderick, P; Chatterjee, N; Chen, J; Heath, AC; Heliövaara, M; Hoft, NR; Hunter, DJ; Jensen, MK; Martin, NG; Montgomery, GW; Niu, T; Payne, TJ; Peltonen, L; Pergadia, ML; Rice, JP; Sherva, R; Spitz, MR; Sun, J; Wang, JC; Weiss, RB; Wheeler, W; Witt, SH; Yang, BZ; Caporaso, NE; Ehringer, MA; Eisen, T; Gapstur, SM; Gelernter, J; Houlston, R; Kaprio, J; Kendler, KS; Kraft, P; Leppert, MF; Li, MD; Madden, PA; Nöthen, MM; Pillai, S; Rietschel, M; Rujescu, D; Schwartz, A; Amos, CI; Bierut, LJ (5 August 2010). "Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD". PLOS Genetics. 6 (8): e1001053. doi:10.1371/journal.pgen.1001053. PMC 2916847. PMID 20700436.

- Fiore, MC; Jaen, CR; Baker, TB; et al. (2008). Treating tobacco use and dependence: 2008 update (PDF). Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service. Archived from the original (PDF) on 2016-03-27. Retrieved 2016-09-02.
- "Anyone Can Become Addicted to Drugs". National Institute on Drug Abuse. July 2015.
- "E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General" (PDF). United States Department of Health and Human Services. Surgeon General of the United States. 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Camenga, Deepa R.; Klein, Jonathan D. (2016). "Tobacco Use Disorders". Child and Adolescent Psychiatric Clinics of North America. 25 (3): 445–460. doi:10.1016/j.chc.2016.02.003. ISSN 1056-4993. PMC 4920978. PMID 27338966.
- Pistillo, Francesco; Clementi, Francesco; Zoli, Michele; Gotti, Cecilia (2015). "Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: Focus on nicotine effects". Progress in Neurobiology. 124: 1–27. doi:10.1016/j.pneurobio.2014.10.002. ISSN 0301-0082. PMID 25447802.
- Shaik, Sabiha Shaheen (2016). "Tobacco Use Cessation and Prevention – A Review". Journal of Clinical and Diagnostic Research. 10 (5): ZE13-7. doi:10.7860/JCDR/2016/19321.7803. ISSN 2249-782X. PMC 4948554. PMID 27437378.
- American Psychiatric Association (22 May 2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. p. 571. ISBN 978-0-89042-557-2.
- Payne, JD; Orellana-Barrios, M; Medrano-Juarez, R; Buscemi, D; Nugent, K (2016). "Electronic cigarettes in the media". Proc (Bayl Univ Med Cent). 29 (3): 280–3. doi:10.1080/08998280.2016.11929436. PMC 4900769. PMID 27365871.
- Besson, Morgane; Forget, Benoît (2016). "Cognitive Dysfunction, Affective States, and Vulnerability to Nicotine Addiction: A Multifactorial Perspective". Frontiers in Psychiatry. 7: 160. doi:10.3389/fpsyt.2016.00160. ISSN 1664-0640. PMC 5030478. PMID 27708591.
 This article incorporates text by Morgane Besson and Benoît Forget available under the CC BY 4.0 license.
This article incorporates text by Morgane Besson and Benoît Forget available under the CC BY 4.0 license. - "Republished: Nicotine and health". BMJ. 349 (nov26 9): 2014.7.0264rep. 2014. doi:10.1136/bmj.2014.7.0264rep. ISSN 1756-1833. PMID 25428425.
- Atta-ur- Rahman; Allen B. Reitz (1 January 2005). Frontiers in Medicinal Chemistry. Bentham Science Publishers. pp. 279–. ISBN 978-1-60805-205-9.
- Martin-Soelch, Chantal (2013). "Neuroadaptive Changes Associated with Smoking: Structural and Functional Neural Changes in Nicotine Dependence". Brain Sciences. 3 (1): 159–176. doi:10.3390/brainsci3010159. ISSN 2076-3425. PMC 4061825. PMID 24961312.
- National Center for Chronic Disease Prevention Health Promotion (US) Office on Smoking Health (2014). "The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, Chapter 5 - Nicotine". Surgeon General of the United States: 107–138. PMID 24455788. Cite journal requires
|journal=(help) - Rowell, Temperance R; Tarran, Robert (2015). "Will Chronic E-Cigarette Use Cause Lung Disease?". American Journal of Physiology. Lung Cellular and Molecular Physiology. 309 (12): L1398–L1409. doi:10.1152/ajplung.00272.2015. ISSN 1040-0605. PMC 4683316. PMID 26408554.
- Benowitz, NL (17 June 2010). "Nicotine addiction". The New England Journal of Medicine. 362 (24): 2295–303. doi:10.1056/NEJMra0809890. PMC 2928221. PMID 20554984.
- Laura J. Martin, David Zieve, Isla Ogilvie, A.D.A.M. Editorial team (7 June 2016). "Nicotine and Tobacco". Medline Plus.CS1 maint: uses authors parameter (link)
- Ware, JJ; van den Bree, MB; Munafò, MR (2011). "Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis". Nicotine & Tobacco Research. 13 (12): 1167–75. doi:10.1093/ntr/ntr118. PMC 3223575. PMID 22071378.
- Yu, Cassie; McClellan, Jon (2016). "Genetics of Substance Use Disorders". Child and Adolescent Psychiatric Clinics of North America. 25 (3): 377–385. doi:10.1016/j.chc.2016.02.002. ISSN 1056-4993. PMID 27338962.
- Wen, L; Yang, Z; Cui, W; Li, M D (2016). "Crucial roles of the CHRNB3–CHRNA6 gene cluster on chromosome 8 in nicotine dependence: update and subjects for future research". Translational Psychiatry. 6 (6): e843. doi:10.1038/tp.2016.103. ISSN 2158-3188. PMC 4931601. PMID 27327258.
- Chen, Li-Shiun; Horton, Amy; Bierut, Laura (2018). "Pathways to precision medicine in smoking cessation treatments". Neuroscience Letters. 669: 83–92. doi:10.1016/j.neulet.2016.05.033. ISSN 0304-3940. PMC 5115988. PMID 27208830.
- Hartmann-Boyce, J; Stead, LF; Cahill, K; Lancaster, T (October 2013). "Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews". Addiction. 108 (10): 1711–21. doi:10.1111/add.12291. PMID 23834141.
- MacDonald, K; Pappa, K (April 2016). "WHY NOT POT?: A Review of the Brain-based Risks of Cannabis". Innov Clin Neurosci. 13 (3–4): 13–22. PMC 4911936. PMID 27354924.
- Hughes, JR; Helzer, JE; Lindberg, SA (8 November 2006). "Prevalence of DSM/ICD-defined nicotine dependence". Drug and Alcohol Dependence. 85 (2): 91–102. doi:10.1016/j.drugalcdep.2006.04.004. PMID 16704909.
- "Current Cigarette Smoking Among Adults — United States, 2005–2013". Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention (63): 1108–1112. 2014.
- Weinberger, AH; Pilver, CE; Mazure, CM; McKee, SA (September 2014). "Stability of smoking status in the US population: a longitudinal investigation". Addiction. 109 (9): 1541–53. doi:10.1111/add.12647. PMC 4127136. PMID 24916157.
- Wadgave, U; Nagesh, L (2016). "Nicotine Replacement Therapy: An Overview". International Journal of Health Sciences. 10 (3): 425–435. doi:10.12816/0048737. PMC 5003586. PMID 27610066.
- "Gender, women, and the tobacco epidemic" (PDF). World Health Organization. 2010.
- DeVito, Elise E.; Krishnan-Sarin, Suchitra (2017). "E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure". Current Neuropharmacology. 15 (4): 438–459. doi:10.2174/1570159X15666171016164430. ISSN 1570-159X. PMC 6018193. PMID 29046158.
- Schraufnagel, Dean E. (2015). "Electronic Cigarettes: Vulnerability of Youth". Pediatric Allergy, Immunology, and Pulmonology. 28 (1): 2–6. doi:10.1089/ped.2015.0490. ISSN 2151-321X. PMC 4359356. PMID 25830075.
- Palazzolo, Dominic L. (November 2013). "Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review". Frontiers in Public Health. 1 (56): 56. doi:10.3389/fpubh.2013.00056. PMC 3859972. PMID 24350225.
- Stratton 2018, p. Chapter 8-52.
- Yoong, Sze Lin; Stockings, Emily; Chai, Li Kheng; Tzelepis, Flora; Wiggers, John; Oldmeadow, Christopher; Paul, Christine; Peruga, Armando; Kingsland, Melanie; Attia, John; Wolfenden, Luke (2018). "Prevalence of electronic nicotine delivery systems (ENDS) use among youth globally: a systematic review and meta-analysis of country level data". Australian and New Zealand Journal of Public Health. 42 (3): 303–308. doi:10.1111/1753-6405.12777. ISSN 1326-0200. PMID 29528527.
External links
| Classification |
|---|
- Fagerstrom Test of Nicotine Dependence (Heatherton et al., 1991)
- Heaviness of Smoking Index (Heatherton et al., 1989)
- Diagnostic and Statistical Manual of Mental Disorders V (DSM-V)
- Tobacco Dependence Screener (Kawakami et al., 1999)
- Nicotine Dependence Syndrome Scale (NDSS; Shiffman, Waters & Hickcox, 2004)
- Cigarette Dependence Scale (Etter et al., 2003)
- Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004)