Molybdenum(V) chloride
Molybdenum(V) chloride is the inorganic compound with the formula [MoCl5]2. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents. Usually called molybdenum pentachloride, it is in fact a dimer with the formula Mo2Cl10.[1]
 | |
-chloride-from-xtal-3D-balls.png.webp) | |
| Names | |
|---|---|
| IUPAC names
Molybdenum(V) chloride Molybdenum pentachloride | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.030.510 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cl10Mo2 | |
| Molar mass | 273.21 g/mol (MoCl5) |
| Appearance | dark-green solid hygroscopic paramagnetic |
| Density | 2.928 g/cm3 |
| Melting point | 194 °C (381 °F; 467 K) |
| Boiling point | 268 °C (514 °F; 541 K) |
| hydrolyzes | |
| Solubility | soluble in dry ether, dry alcohol, organic solvents |
| Structure | |
| monoclinic | |
| edge-shared bioctahedron | |
| Hazards | |
| Main hazards | oxidizer, hydrolyzes to release HCl |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Molybdenum(V) fluoride Molybdenum(IV) bromide Molybdenum(III) iodide |
Other cations |
Chromium(IV) chloride Tungsten(V) chloride |
Related molybdenum chlorides |
Molybdenum(II) chloride Molybdenum(III) chloride Molybdenum(IV) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
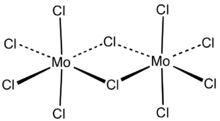
Structure
Each molybdenum has local octahedral symmetry and two chlorides bridge between the molybdenum centers.[2] A similar structure is also found for the pentachlorides of W, Nb and Ta.[3] In the gas phase and partly in solution, the dimers partially dissociates to give a monomeric pentahalide.[4] The monomer is paramagnetic, with one unpaired electron per Mo center, reflecting the fact that the formal oxidation state is +5, leaving one valence electron on the metal center.
Preparation and properties
MoCl5 is prepared by chlorination of Mo metal but also chlorination of MoO3. The unstable hexachloride MoCl6 is not produced in this way.[5]
MoCl5 is reduced by acetonitrile to afford an orange acetonitrile complex, MoCl4(MeCN)2. This complex in turn reacts with THF to give MoCl4(THF)2, a precursor to other molybdenum-containing complexes.[6]
Molybdenum(IV) bromide is prepared by treatment of molybdenum(V) chloride with hydrogen bromide:
- 2 MoCl5 + 10 HBr → 2 MoBr4 + 10 HCl + Br2
The reaction proceeds via the unstable molybdenum(V) bromide, which releases bromine at room temperature.[7]
MoCl5 is a good Lewis acid toward non-oxidizable ligands. It forms an adduct with chloride to form [MoCl6]−. In organic synthesis, the compound finds occasional use in chlorinations, deoxygenation, and oxidative coupling reactions.[8]
Molybdenum hexachloride
Molybdenum(VI) chloride is known, but it cannot be prepared by addition of chlorine to MoCl5.[9]
Safety considerations
MoCl5 is an aggressive oxidant and readily hydrolyzes to release HCl.
References
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego, CA: Academic Press. ISBN 0-12-352651-5.
- Beck, J.; Wolf, F. (1997). "Three New Polymorphic Forms of Molybdenum Pentachloride". Acta Crystallogr. B53 (6): 895–903. doi:10.1107/S0108768197008331.
- Wells, A. E. (1984). Structural Inorganic Chemistry (5th ed.). Oxford: Clarendon Press.
- Brunvoll, J.; Ischenko, A. A.; Spiridonov, V. P.; Strand, T. G. (1984). "Composition and Molecular Structure of Gaseous Molybdenum Pentachloride by Electron Diffraction". Acta Chem. Scand. A38: 115–120. doi:10.3891/acta.chem.scand.38a-0115.
- Tamadon, Farhad; Seppelt, Konrad (2013). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angew. Chem. Int. Ed. 52 (2): 767–769. doi:10.1002/anie.201207552. PMID 23172658.
- Dilworth, Jonathan R.; Richards, Raymond L. (1990). "The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes". Inorganic Syntheses. Inorganic Syntheses. 28. pp. 33–43. doi:10.1002/9780470132593.ch7. ISBN 9780470132593.
- Calderazzo, Fausto; Maichle-Mössmer, Cäcilie; Pampaloni, Guido; Strähle, Joachim (1993). "Low-Temperature Syntheses of Vanadium(III) and Molybdenum(IV) Bromides by Halide Exchange". J. Chem. Soc., Dalton Trans. (5): 655–658. doi:10.1039/DT9930000655.
- Kauffmann, T.; Torii, S.; Inokuchi, T. (2004). "Molybdenum(V) Chloride". Encyclopedia of Reagents for Organic Synthesis. New York, NY: J. Wiley & Sons. doi:10.1002/047084289X. ISBN 9780471936237.
- Tamadon, Farhad; Seppelt, K. (2012). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angewandte Chemie International Edition. 52 (2): 767–769. doi:10.1002/anie.201207552. PMID 23172658.CS1 maint: uses authors parameter (link)