Molybdenum(III) chloride
Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Molybdenum(III) chloride Molybdenum trichloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.418 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| MoCl3 | |||
| Molar mass | 202.30 g/mol | ||
| Appearance | dark red solid paramagnetic | ||
| Density | 3.58 g/cm3 | ||
| Melting point | 410 °C (770 °F; 683 K) (decomposes) | ||
| insoluble | |||
| Solubility | insoluble in ethanol, diethyl ether | ||
| +43.0·10−6 cm3/mol | |||
| Hazards | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
Molybdenum(III) fluoride Molybdenum(III) bromide Molybdenum(III) iodide | ||
Other cations |
Chromium(IV) chloride Tungsten(V) chloride | ||
Related molybdenum chlorides |
Molybdenum(II) chloride Molybdenum(IV) chloride Molybdenum(V) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis and structure
Molybdenum(III) chloride is synthesized by the reduction of molybdenum(V) chloride with hydrogen.[2] A higher yield is produced by the reduction of pure molybdenum(V) chloride with anhydrous tin(II) chloride as the reducing agent.[3]
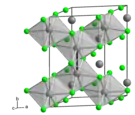
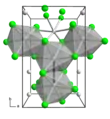
Molybdenum trichloride exists as two polymorphs: alpha (α) and beta (β). The alpha structure is similar to that of aluminum chloride (AlCl3). In this structure, molybdenum has octahedral coordination geometry and exhibits cubic close-packing in its crystalline structure. The beta structure, however, exhibits hexagonal close packing.[4]
THF complex
Molybdenum trichloride gives a THF complex MoCl3(thf)3. This pale orange solid is synthesized by reducing a THF solution of MoCl4(THF)2 with tin powder. The complex has octahedral geometry. The IR spectrum is free of intense bands in the 900–1000 cm−1, a characteristic of molybdenum oxo species.[5]
Hexa(tert-butoxy)dimolybdenum(III) is prepared by the salt metathesis reaction from MoCl3(thf)3:[6]
- 2 MoCl3(thf)3 + 6 LiOBu-t → Mo2(OBu-t)6 + 6 LiCl + 6 thf
References
- Perry DL (2011). Handbook of Inorganic Compounds (2nd ed.). Boca Raton: Taylor & Francis. p. 279. ISBN 978-1-4398-1461-1.
- Couch DE, Brenner A (1959). "Preparation of Trichloride and Tetrachloride of Molybdenum". Journal of Research of the National Bureau of Standards. Section A, Physics and Chemistry. 63A (2): 185–188. doi:10.6028/jres.063A.013. PMC 5287202. PMID 31216151.
- Larson ML (1970). "Preparation of Some Metal Halides- Anhydrous Molybdenum Halides and Oxide Halides - A Summary". Inorganic Syntheses, Volume 12. pp. 178–181.
- Hillebrecht H, Schmidt PJ, Rotter HW, Thiele G, Zönnchen P, Bengel H, Cantow HJ, Magonov SN, Whangbo MH (1997). "Structural and scanning microscopy studies of layered compounds MCl3 (M= Mo, Ru, Cr) and MOCl2 (M= V, Nb, Mo, Ru, Os)". Journal of Alloys and Compounds. 246 (1–2): 70–79. doi:10.1016/S0925-8388(96)02465-6.
- Dilworth, Jonathan R.; Richards, Raymond L. (1990). "The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes". Inorganic Syntheses. 28: 33–43. doi:10.1002/9780470132593.ch7.
- Broderick, Erin M.; Browne, Samuel C.; Johnson, Marc J. A. (2014). "Dimolybdenum and Ditungsten Hexa(Alkoxides)". Inorganic Syntheses. 36: 95–102. doi:10.1002/9781118744994.ch18.
Further reading
- Dilworth JR, Richards RL, Chen GJ, Mcdonald JW (January 1990). The Synthesis of Molybdenum and Tungsten Dinitrogen Complexes. Inorganic Syntheses: Reagents for Transition Metal Complex and Organometallic Syntheses. 28. pp. 33–43.