PCM1
Pericentriolar material 1, also known as PCM1, is a protein which in humans is encoded by the PCM1 gene.[4][5][6]
Function
The PCM1 protein was originally identified by virtue of its distinct cell cycle-dependent association with the centrosome complex and microtubules. The protein appears to associate with the centrosome complex during the cell cycle. Dissociation occurs during mitosis when PCM1 is dispersed throughout the cell. Immunolabeling studies performed found that PCM1 was present in centriolar satellites and in electron dense granules between 70 and 100 nm in diameter. These were originally thought to be scattered only around the centrosomes, but further studies proved that PCM1 was also found throughout the cytoplasm.
PCM1 was shown to be essential for cell division because PCM1 antibodies cause cell-cycle arrest when microinjected into fertilized murine eggs. Targeting of centrin, pericentrin and ninein was also dramatically reduced after PCM1 depletion using siRNA, overexpression of PCM1 deletion mutants and PCM1 antibody microinjection.[7] As a result of this depletion, the radial organization of the microtubules was found to be disrupted, but did not appear to effect microtubule nucleation.
Structure
PCM1 has four known transcripts, the longest of which has 39 exons. The open reading frame of PCM1 encodes a protein of 2024 amino acids. The protein contains coiled coil regions between areas of low complexity as well as an adenosine triphosphate (ATP) / GTPase domain, a nuclear localization domain and a eukaryotic molybdopterin domain. The eukaryotic molybdopterin binding domain is currently found in only five other human genes, xanthine dehydrogenase, sulfite oxidase (mitochondrial precursor), aldehyde oxidase, erythropoietin receptor precursor and the ATPbinding cassette, sub-family A, member 2 (ABCA2).
Tissue distribution
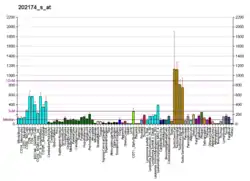
PCM1 mRNA expression in the mouse brain has been found to be highest in the hippocampus.[8] In humans it is expressed above the median level of central nervous system (CNS) expression in most parts of the brain.[9]
Clinical significance
Mutations in the PCM1 gene have been shown to cause genetic susceptibility to schizophrenia. If an isoleucine amino acid change in PCM1 is inherited the risk of developing schizophrenia was found to be 68% in two independent samples from south England and Scotland. This means that it may now be possible to offer very limited genetic counselling to a small proportion of people with schizophrenia who are also carriers of this mutation.[10][11]
PCM1 forms a complex at the centrosome with disrupted-in-schizophrenia 1 (DISC1) and Bardet-Biedl syndrome 4 protein (BBS4), which provides a link between aberrant PCM1 and the abnormal cortical development associated with the pathology of schizophrenia.[12]
References
- GRCm38: Ensembl release 89: ENSMUSG00000031592 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: PCM1 pericentriolar material 1".
- Balczon R, Bao L, Zimmer WE (March 1994). "PCM-1, A 228-kD centrosome autoantigen with a distinct cell cycle distribution". J. Cell Biol. 124 (5): 783–93. doi:10.1083/jcb.124.5.783. PMC 2119948. PMID 8120099.
- Hames RS, Crookes RE, Straatman KR, Merdes A, Hayes MJ, Faragher AJ, Fry AM (April 2005). "Dynamic Recruitment of Nek2 Kinase to the Centrosome Involves Microtubules, PCM-1, and Localized Proteasomal Degradation". Mol. Biol. Cell. 16 (4): 1711–24. doi:10.1091/mbc.E04-08-0688. PMC 1073654. PMID 15659651.
- Dammermann, A.; Merdes, A. (2002). "Assembly of centrosomal proteins and microtubule organization depends on PCM-1". The Journal of Cell Biology. 159 (2): 255–266. doi:10.1083/jcb.200204023. PMC 2173044. PMID 12403812.
- "Gene Expression Summary for Pcm1; pericentriolar material 1". Allen Institute for Brain Science. Retrieved 2009-04-30.
- "PCM1, Probe set 202174_s_at". BioGPS - your Gene Portal System. Retrieved 2009-04-30.
- Datta SR, McQuillin A, Rizig M, Blaveri E, Thirumalai S, Kalsi G, Lawrence J, Bass NJ, Puri V, Choudhury K, Pimm J, Crombie C, Fraser G, Walker N, Curtis D, Zvelebil M, Pereira A, Kandaswamy R, St Clair D, Gurling HM (December 2008). "A threonine to isoleucine missense mutation in the pericentriolar material 1 gene is strongly associated with schizophrenia". Mol. Psychiatry. 15 (6): 615–28. doi:10.1038/mp.2008.128. PMID 19048012.
- Gurling HM, Critchley H, Datta SR, McQuillin A, Blaveri E, Thirumalai S, Pimm J, Krasucki R, Kalsi G, Quested D, Lawrence J, Bass N, Choudhury K, Puri V, O'Daly O, Curtis D, Blackwood D, Muir W, Malhotra AK, Buchanan RW, Good CD, Frackowiak RS, Dolan RJ (August 2006). "Genetic Association and Brain Morphology Studies and the Chromosome 8p22 Pericentriolar Material 1 (PCM1) Gene in Susceptibility to Schizophrenia". Arch. Gen. Psychiatry. 63 (8): 844–54. doi:10.1001/archpsyc.63.8.844. PMC 2634866. PMID 16894060.
- Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, Kubo A, Tsukita S, Pulver AE, Nakajima K, Cascella NG, Katsanis N, Sawa A (September 2008). "PCM1 is recruited to the centrosome by the cooperative action of DISC1 and BBS4 and is a candidate for psychiatric illness". Arch. Gen. Psychiatry. 65 (9): 996–1006. doi:10.1001/archpsyc.65.9.996. PMC 2727928. PMID 18762586.
- Li, Q; Hansen D; Killilea A; Joshi H C; Palazzo R E; Balczon R (February 2001). "Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1". J. Cell Sci. 114 (Pt 4): 797–809. ISSN 0021-9533. PMID 11171385.
Further reading
- Tollenaere M.A.X.; Mailand N; Bekker-Jensen S (2014). "Centriolar satellites: key mediators of centrosome functions". Cell. Mol. Life Sci. 72 (1): 11–23. doi:10.1007/s00018-014-1711-3. PMID 25173771. S2CID 2243856.
- Ohata H, Fujiwara Y, Koyama K, Nakamura Y (1995). "Mapping of the human autoantigen pericentriolar material 1 (PCM1) gene to chromosome 8p21.3-p22". Genomics. 24 (2): 404–6. doi:10.1006/geno.1994.1640. PMID 7698772.
- Balczon R, Bao L, Zimmer WE (1994). "PCM-1, A 228-kD centrosome autoantigen with a distinct cell cycle distribution". J. Cell Biol. 124 (5): 783–93. doi:10.1083/jcb.124.5.783. PMC 2119948. PMID 8120099.
- Engelender S, Sharp AH, Colomer V, et al. (1998). "Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin". Hum. Mol. Genet. 6 (13): 2205–12. doi:10.1093/hmg/6.13.2205. PMID 9361024.
- Dias Neto E, Correa RG, Verjovski-Almeida S, et al. (2000). "Shotgun sequencing of the human transcriptome with ORF expressed sequence tags". Proc. Natl. Acad. Sci. U.S.A. 97 (7): 3491–6. Bibcode:2000PNAS...97.3491D. doi:10.1073/pnas.97.7.3491. PMC 16267. PMID 10737800.
- Corvi R, Berger N, Balczon R, Romeo G (2000). "RET/PCM-1: a novel fusion gene in papillary thyroid carcinoma". Oncogene. 19 (37): 4236–42. doi:10.1038/sj.onc.1203772. PMID 10980597.
- Li Q, Hansen D, Killilea A, et al. (2001). "Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1". J. Cell Sci. 114 (Pt 4): 797–809. PMID 11171385.
- Kumar S, Connor JR, Dodds RA, et al. (2001). "Identification and initial characterization of 5000 expressed sequenced tags (ESTs) each from adult human normal and osteoarthritic cartilage cDNA libraries". Osteoarthr. Cartil. 9 (7): 641–53. doi:10.1053/joca.2001.0421. PMID 11597177.
- Dammermann A, Merdes A (2002). "Assembly of centrosomal proteins and microtubule organization depends on PCM-1". J. Cell Biol. 159 (2): 255–66. doi:10.1083/jcb.200204023. PMC 2173044. PMID 12403812.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Ansley SJ, Badano JL, Blacque OE, et al. (2003). "Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome". Nature. 425 (6958): 628–33. Bibcode:2003Natur.425..628A. doi:10.1038/nature02030. PMID 14520415. S2CID 4310157.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Kim JC, Badano JL, Sibold S, et al. (2004). "The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression". Nat. Genet. 36 (5): 462–70. doi:10.1038/ng1352. PMID 15107855.
- Brill LM, Salomon AR, Ficarro SB, et al. (2004). "Robust phosphoproteomic profiling of tyrosine phosphorylation sites from human T cells using immobilized metal affinity chromatography and tandem mass spectrometry". Anal. Chem. 76 (10): 2763–72. doi:10.1021/ac035352d. PMID 15144186.
- Armes JE, Hammet F, de Silva M, et al. (2004). "Candidate tumor-suppressor genes on chromosome arm 8p in early-onset and high-grade breast cancers". Oncogene. 23 (33): 5697–702. doi:10.1038/sj.onc.1207740. PMID 15184884.
- Beausoleil SA, Jedrychowski M, Schwartz D, et al. (2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proc. Natl. Acad. Sci. U.S.A. 101 (33): 12130–5. Bibcode:2004PNAS..10112130B. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Ballif BA, Villén J, Beausoleil SA, et al. (2005). "Phosphoproteomic analysis of the developing mouse brain". Mol. Cell. Proteomics. 3 (11): 1093–101. doi:10.1074/mcp.M400085-MCP200. PMID 15345747.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Hames RS, Crookes RE, Straatman KR, et al. (2005). "Dynamic Recruitment of Nek2 Kinase to the Centrosome Involves Microtubules, PCM-1, and Localized Proteasomal Degradation". Mol. Biol. Cell. 16 (4): 1711–24. doi:10.1091/mbc.E04-08-0688. PMC 1073654. PMID 15659651.
- Reiter A, Walz C, Watmore A, et al. (2005). "The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2". Cancer Res. 65 (7): 2662–7. doi:10.1158/0008-5472.CAN-04-4263. PMID 15805263.
- Murati A, Gelsi-Boyer V, Adélaïde J, et al. (2005). "PCM1-JAK2 fusion in myeloproliferative disorders and acute erythroid leukemia with t(8;9) translocation". Leukemia. 19 (9): 1692–6. doi:10.1038/sj.leu.2403879. PMID 16034466.