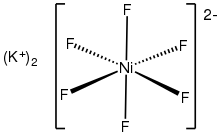
Potassium hexafluoronickelate(IV)
Potassium hexafluoronickelate(IV) is an inorganic compound with the chemical formula K
2NiF
6. It can be produced through the reaction of potassium fluoride, nickel dichloride, and fluorine.
 | |
| Names | |
|---|---|
| IUPAC name
potassium hexafluoronickelate(IV) | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.153.655 |
| EC Number |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| K2NiF6 | |
| Molar mass | 250.880 |
| Hazards[1] | |
| Safety data sheet | External SDS |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H302, H312, H317, H331, H350 | |
| P201, P261, P280, P304+340, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It reacts violently with water, releasing oxygen. It dissolves in anhydrous hydrogen fluoride to produce a light-red solution. Potassium hexafluoronickelate(IV) decomposes at 350 °C, forming potassium hexafluoronickelate(III), nickel(II) fluoride, and fluorine:[2]
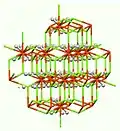
Chemical structure of solid K2NiF6 as determined by X-ray crystallography.
Potassium hexafluoronickelate is a strong oxidant. It can turn chlorine pentafluoride and bromine pentafluoride into ClF+
6 and BrF+
6, respectively:[3]
- ( X = Cl or Br , -60 °C , aHF = anhydrous hydrogen fluoride).
It adopts the structure seen for K2PtCl6 and Mg2FeH6.[4]
References
- "Potassium Hexafluoronickelate(IV)". American Elements. Retrieved December 19, 2018.
- (in Chinese)张青莲. 《无机化学丛书》第九卷:锰分族、铁系、铂系. 北京: 科学出版社. pp. P333. ISBN 7-03-002238-6.
- Schroer, Thorsten; Christe, Karl O. (2001). "Novel Synthesis of ClF6+ and BrF6+ Salts". Inorganic Chemistry. 40 (10): 2415–9. doi:10.1021/ic001024. PMID 11327921.
- Taylor, J. C. "A comparison of profile decomposition and Rietveld methods for structurtal refinement with powder diffraction data" Zeitschrift für Kristallographie 1987, volume 181, p151-160.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.