Nickel(II) bromide
Nickel(II) bromide is the name for the inorganic compounds with the chemical formula NiBr2(H2O)x. The value of x can be 0 for the anhydrous material, as well as 2, 3, or 6 for the three known hydrate forms. The anhydrous material is a yellow-brown solid which dissolves in water to give blue-green hexahydrate (see picture).
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Nickel(II) bromide | |
| Other names
Nickel dibromide, Nickel bromide, Nickelous bromide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.318 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NiBr2 | |
| Molar mass | 218.53 g/mol |
| Appearance | yellow-brown crystals |
| Odor | odorless |
| Density | 5.098 g/cm3[1] |
| Melting point | 963 °C (1,765 °F; 1,236 K) sublimes |
| 113 g/100ml (0 °C) 122 g/100ml (10 °C) 134 g/100ml (25 °C)[2] 144 g/100ml (40 °C) 155 g/100ml (100 °C) | |
| +5600.0·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Irritant, corrosive |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
nickel(II) fluoride nickel(II) chloride nickel(II) iodide |
Other cations |
cobalt(II) bromide copper(II) bromide palladium(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
The structure of the nickel bromides varies with the degree of hydration. In all of these cases, the nickel(II) ion adopts an octahedral molecular geometry.
- Anhydrous NiBr2 adopts the cadmium chloride structure.[3][4] The interatomic distance for Ni-Br is 2.52—2.58 Å.[4]
- The structure of the trihydrate has not been confirmed by X-ray crystallography. It is assumed to adopt a chain structure.[5]
- The di- and hexahydrates adopt structures akin to those for the corresponding chlorides. The dihydrate consists of a linear chain, whereas the hexahydrate features isolated trans-[NiBr2(H2O)4] molecules together with two noncoordinated water molecules.
Reactions and uses
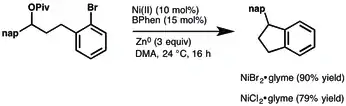
NiBr2 has Lewis acid character. NiBr2 is also used to prepare catalysts for cross-coupling reactions and various carbonylations.[3]
Empirically, NiBr2-glyme has shown increased activity compared to that of NiCl2-glyme for some transformations.[6]
Safety
Nickel(II) is toxic and suspected to be a cancer agent.[3]
References
- http://chemdat.merck.de/documents/sds/emd/deu/de/8181/818174.pdf
- http://chemister.ru/Database/properties-en.php?dbid=1&id=3859
- Luh, Tien-Yau; Kuo, Chi-Hong (2001-01-01). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289X.rn009. ISBN 9780470842898.
- Wakita, Hisanobu; Ichihashi, Mitsuyoshi; Mibuchi, Takeharu; Masuda, Isao (1982-03-01). "The Structure of Nickel(II) Bromide in Highly Concentrated Aqueous Solution by X-Ray Diffraction Analysis". Bulletin of the Chemical Society of Japan. 55 (3): 817–821. doi:10.1246/bcsj.55.817. ISSN 0009-2673.
- DeFotis, G. C.; Goodey, J. R.; Narducci, A. A.; Welch, M. H. "NiBr2·3H2O, a lower dimensional antiferromagnet" Journal of Applied Physics (1996), 79(8, Pt. 2A), 4718-4720. doi:10.1063/1.361651
- Konev, Mikhail O.; Hanna, Luke E.; Jarvo, Elizabeth R. (2016-06-01). "Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides". Angewandte Chemie International Edition. 55 (23): 6730–6733. doi:10.1002/anie.201601206. PMID 27099968.
