Nickel(II) sulfate
Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble blue green coloured salt is a common source of the Ni2+ ion for electroplating.
| |||
6SO4.png.webp) | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nickel(II) sulfate | |||
| Other names
Nickelous sulfate | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.186 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII |
| ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| NiSO4 | |||
| Molar mass | 154.75 g/mol (anhydrous) 262.85 g/mol (hexahydrate) 280.86 g/mol (heptahydrate) | ||
| Appearance | yellow solid (anhydrous) blue crystals (hexahydrate) green-blue crystals (heptahydrate) | ||
| Odor | odorless | ||
| Density | 4.01 g/cm3 (anhydrous) 2.07 g/cm3 (hexahydrate) 1.948 g/cm3 (heptahydrate) | ||
| Melting point | > 100 °C (anhydrous) 53 °C (hexahydrate) | ||
| Boiling point | 840 °C (1,540 °F; 1,110 K) (anhydrous, decomposes) 100 °C (hexahydrate, decomposes) | ||
| 65 g/100 mL (20 °C) 77.5 g/100 mL (30 °C) (heptahydrate) | |||
| Solubility | anhydrous insoluble in ethanol, ether, acetone hexahydrate insoluble in ethanol, ammonia heptahydrate soluble in alcohol | ||
| Acidity (pKa) | 4.5 (hexahydrate) | ||
| +4005.0·10−6 cm3/mol | |||
Refractive index (nD) |
1.511 (hexahydrate) 1.467 (heptahydrate) | ||
| Structure | |||
| cubic (anhydrous) tetragonal (hexahydrate) rhombohedral (hexahydrate) | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
EU classification (DSD) (outdated) |
Carc. Cat. 1 Muta. Cat. 3 Repr. Cat. 2 Toxic (T) Harmful (Xn) Irritant (Xi) Dangerous for the environment (N) | ||
| R-phrases (outdated) | R49, R61, R20/22, R38, R42/43, R48/23, R68, R50/53 | ||
| S-phrases (outdated) | S53, S45, S60, S61 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
264 mg/kg | ||
| Related compounds | |||
Other cations |
Cobalt(II) sulfate Copper(II) sulfate Iron(II) sulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Approximately 40,000 tonnes were produced in 2005. It is mainly used for electroplating of nickel.[1]
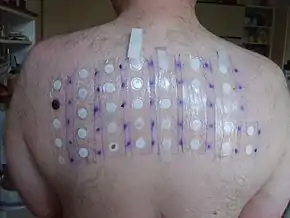
In 2005–06, nickel sulfate was the top allergen in patch tests (19.0%).[2]
Structures
.jpg.webp)
At least seven sulfate salts of nickel(II) are known. These salts differ in terms of their hydration or crystal habit.
The common tetragonal hexahydrate crystallizes from aqueous solution between 30.7 and 53.8 °C. Below these temperatures, a heptahydrate crystallises, and above these temperatures an orthorhombic hexahydrate forms. The yellow anhydrous form, NiSO4, is a high melting solid that is rarely encountered in the laboratory. This material is produced by heating the hydrates above 330 °C. It decomposes at still higher temperatures to nickel oxide.[1]
X-ray crystallography measurements show that NiSO4·6H2O consists of the octahedral [Ni(H2O)6]2+ ions. These ions in turn are hydrogen bonded to sulfate ions.[3] Dissolution of the salt in water gives solutions containing the aquo complex [Ni(H2O)6]2+.
All nickel sulfates are paramagnetic.
Production, applications, and coordination chemistry
The salt is usually obtained as a by-product of copper refining. It is also produced by dissolution of nickel metal or nickel oxides in sulfuric acid.
Aqueous solutions of nickel sulfate reacts with sodium carbonate to precipitate nickel carbonate, a precursor to nickel-based catalysts and pigments.[4] Addition of ammonium sulfate to concentrated aqueous solutions of nickel sulfate precipitates Ni(NH4)2(SO4)2·6H2O. This blue-coloured solid is analogous to Mohr's salt, Fe(NH4)2(SO4)2·6H2O.[1]
Nickel sulfate is used in the laboratory. Columns used in polyhistidine-tagging, useful in biochemistry and molecular biology, are regenerated with nickel sulfate. Aqueous solutions of NiSO4·6H2O and related hydrates react with ammonia to give [Ni(NH3)6]SO4 and with ethylenediamine to give [Ni(H2NCH2CH2NH2)3]SO4. The latter is occasionally used as a calibrant for magnetic susceptibility measurements because it has no tendency to hydrate.
Natural occurrence
Nickel sulfate occurs as the rare mineral retgersite, which is a hexahydrate. The second hexahydrate is known as nickel hexahydrite (Ni,Mg,Fe)SO4·6H2O. The heptahydrate, which is relatively unstable in air, occurs as morenosite. The monohydrate occurs as very rare mineral dwornikite (Ni,Fe)SO4·H2O.
Safety
In 2005–06, nickel sulfate was the top allergen in patch tests (19.0%).[2] Nickel sulfate is classified as a human carcinogen[5][6][7][8] based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers.[9] In a 2-year inhalation study in F344 rats and B6C3F1 mice, there was no evidence of carcinogenic activity, although increased lung inflammations and bronchial lymph node hyperplasia were observed.[10] These results strongly suggest that there is a threshold for the carcinogenicity of nickel sulfate via inhalation. In a 2-year study with daily oral administration of nickel sulfate hexahydrate to F344 rats, no evidence for increased carcinogenic activity was observed.[11] The human and animal data consistently indicate a lack of carcinogenicity via the oral route of exposure and limit the carcinogenicity of nickel compounds to respiratory tumours after inhalation.[12] Whether these effects are relevant to humans is unclear as epidemiological studies of highly exposed female workers have not shown adverse developmental toxicity effects.[13][14][15][16]
References
- K. Lascelles, L. G. Morgan, D. Nicholls, D. Beyersmann “Nickel Compounds” in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Vol. A17 p. 235 doi:10.1002/14356007.a17_235.pub2.
- Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60.
- Wells, A. F. (1984). Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- H. B. W. Patterson, "Catalysts" in Hydrogenation of Fats and Oils G. R. List and J. W. King, Eds., 1994, AOCS Press, Urbana.
- IARC (2012). “Nickel and nickel compounds” IARC Monogr Eval Carcinog Risks Hum, Volume 100C: 169-218.
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 [OJ L 353, 31.12.2008, p. 1]. Annex VI. www.eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32008R1272 Accessed July 13, 2017.
- Globally Harmonised System of Classification and Labelling of Chemicals (GHS), Fifth revised edition, United Nations, New York and Geneva, 2013. PDF at https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev05/English/ST-SG-AC10-30-REv5e.pdf Accessed July 13, 2017.
- NTP (National Toxicology Program). 2016. “Report on Carcinogens”, 14th Edition.; Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html Accessed July 13, 2017.
- International Committee on Nickel Carcinogenesis in Man (ICNCM). (1990). Report of the International Committee on Nickel Carcinogenesis in Man. Scan. J. Work Environ. Health. 16(1): 1-82.
- National Toxicology Program (NTP). (1996). Toxicology and Carcinogenesis Studies of Nickel Sulfate Hexahydrate (CAS NO. 10101-97-0) in F344/N Rats and B6CF1 Mice (Inhalation Studies). US DHHS. NTP TR 454. NIH Publication No. 96-3370.
- Heim, K. E.; Bates, H. K.; Rush, R. E.; Oller, A. R. (2007). “Oral Carcinogenicity Study with Nickel Sulfate Hexahydrate in Fischer 344 Rats.” Toxicol. Appl. Pharmacol. 224(2): 126-137.
- Cogliano, V. J.; Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F. E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; Wild, C. P. (2011). “Preventable Exposures Associated With Human Cancers”. J Natl Cancer Inst 103: 1827-1839.
- Vaktskjold, A.; Talykova, L. V.; Chashchin, V. P.; Odland, J. O.; Nieboer, E. (2008). “Spontaneous abortions among nickel-exposed female refinery workers.” Int J Environ Health Res. 18(2): 99-115.
- Vaktskjold, A.; Talykova, L. V.; Chashchin, V. P.; Nieboer, E.; Thomassen, Y.; Odland, J. O. (2006). “Genital malformations in newborns of female nickel-refinery workers.” Scan J Work Environ Health. 32(1): 41-50.
- Vaktskjold, A.; Talykova, L. V.; Chashchin, V. P.; Odland, J. O.; Nieboer, E. (2007). “Small-for-gestational-age newborns of female refinery workers exposed to nickel.” Int J Occup Med Environ Health. 20(4): 327-38.
- Vaktskjold, A.; Talykova, L. V.; Chashchin, V. P.; Odland, J. O.; Nieboer, E. (2008). “Maternal nickel exposure and congenital musculoskeletal defects.” Am J Ind Med. 51(11): 825-33.