Randomized experiment
In science, randomized experiments are the experiments that allow the greatest reliability and validity of statistical estimates of treatment effects. Randomization-based inference is especially important in experimental design and in survey sampling.
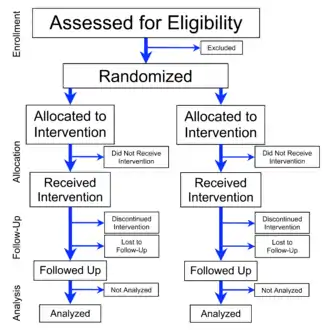
Overview
In the statistical theory of design of experiments, randomization involves randomly allocating the experimental units across the treatment groups. For example, if an experiment compares a new drug against a standard drug, then the patients should be allocated to either the new drug or to the standard drug control using randomization.
Randomized experimentation is not haphazard. Randomization reduces bias by equalising other factors that have not been explicitly accounted for in the experimental design (according to the law of large numbers). Randomization also produces ignorable designs, which are valuable in model-based statistical inference, especially Bayesian or likelihood-based. In the design of experiments, the simplest design for comparing treatments is the "completely randomized design". Some "restriction on randomization" can occur with blocking and experiments that have hard-to-change factors; additional restrictions on randomization can occur when a full randomization is infeasible or when it is desirable to reduce the variance of estimators of selected effects.
Randomization of treatment in clinical trials pose ethical problems. In some cases, randomization reduces the therapeutic options for both physician and patient, and so randomization requires clinical equipoise regarding the treatments.
Online randomized controlled experiments
Web sites can run randomized controlled experiments [2] to create a feedback loop.[3] Key differences between offline experimentation and online experiments include:[3][4]
- Logging: user interactions can be logged reliably.
- Number of users: large sites, such as Amazon, Bing/Microsoft, and Google run experiments, each with over a million users.
- Number of concurrent experiments: large sites run tens of overlapping, or concurrent, experiments.[5]
- Robots, whether web crawlers from valid sources or malicious internet bots.
- Ability to ramp-up experiments from low percentages to higher percentages.
- Speed / performance has significant impact on key metrics.[3][6]
- Ability to use the pre-experiment period as an A/A test to reduce variance.[7]
History
A controlled experiment appears to have been suggested in the Old Testament's Book of Daniel. King Nebuchadnezzar proposed that some Israelites eat "a daily amount of food and wine from the king's table." Daniel preferred a vegetarian diet, but the official was concerned that the king would "see you looking worse than the other young men your age? The king would then have my head because of you." Daniel then proposed the following controlled experiment: "Test your servants for ten days. Give us nothing but vegetables to eat and water to drink. Then compare our appearance with that of the young men who eat the royal food, and treat your servants in accordance with what you see". (Daniel 1, 12– 13).[8][9]
Randomized experiments were institutionalized in psychology and education in the late eighteen-hundreds, following the invention of randomized experiments by C. S. Peirce.[10][11][12][13] Outside of psychology and education, randomized experiments were popularized by R.A. Fisher in his book Statistical Methods for Research Workers, which also introduced additional principles of experimental design.
Statistical interpretation
The Rubin Causal Model provides a common way to describe a randomized experiment. While the Rubin Causal Model provides a framework for defining the causal parameters (i.e., the effects of a randomized treatment on an outcome), the analysis of experiments can take a number of forms. Most commonly, randomized experiments are analyzed using ANOVA, student's t-test, regression analysis, or a similar statistical test.
Empirical evidence that randomization makes a difference
Empirically differences between randomized and non-randomized studies,[14] and between adequately and inadequately randomized trials have been difficult to detect.[15][16]
See also
References
- Schulz KF, Altman DG, Moher D; for the CONSORT Group (2010). "CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials". BMJ. 340: c332. doi:10.1136/bmj.c332. PMC 2844940. PMID 20332509.CS1 maint: multiple names: authors list (link)
- Kohavi, Ron; Longbotham, Roger (2015). "Online Controlled Experiments and A/B Tests" (PDF). In Sammut, Claude; Webb, Geoff (eds.). Encyclopedia of Machine Learning and Data Mining. Springer. pp. to appear.
- Kohavi, Ron; Longbotham, Roger; Sommerfield, Dan; Henne, Randal M. (2009). "Controlled experiments on the web: survey and practical guide". Data Mining and Knowledge Discovery. 18 (1): 140–181. doi:10.1007/s10618-008-0114-1. ISSN 1384-5810.
- Kohavi, Ron; Deng, Alex; Frasca, Brian; Longbotham, Roger; Walker, Toby; Xu Ya (2012). "Trustworthy Online Controlled Experiments: Five Puzzling Outcomes Explained". Proceedings of the 18th ACM SIGKDD Conference on Knowledge Discovery and Data Mining.
- Kohavi, Ron; Deng Alex; Frasca Brian; Walker Toby; Xu Ya; Nils Pohlmann (2013). Online Controlled Experiments at Large Scale. Proceedings of the 19th ACM SIGKDD Conference on Knowledge Discovery and Data Mining. 19. Chicago, Illinois, USA: ACM. pp. 1168–1176. doi:10.1145/2487575.2488217.
- Kohavi, Ron; Deng Alex; Longbotham Roger; Xu Ya (2014). Seven Rules of Thumb for Web Site Experimenters. Proceedings of the 20th ACM SIGKDD Conference on Knowledge Discovery and Data Mining. 20. New York, New York, USA: ACM. pp. 1857–1866. doi:10.1145/2623330.2623341.
- Deng, Alex; Xu, Ya; Kohavi, Ron; Walker, Toby (2013). "Improving the Sensitivity of Online Controlled Experiments by Utilizing Pre-Experiment Data". WSDM 2013: Sixth ACM International Conference on Web Search and Data Mining.
- Neuhauser, D; Diaz, M (2004). "Daniel: using the Bible to teach quality improvement methods". Quality and Safety in Health Care. 13 (2): 153–155. doi:10.1136/qshc.2003.009480. PMC 1743807. PMID 15069225.
- Angrist, Joshua; Pischke Jörn-Steffen (2014). Mastering 'Metrics: The Path from Cause to Effect. Princeton University Press. p. 31.
- Charles Sanders Peirce and Joseph Jastrow (1885). "On Small Differences in Sensation". Memoirs of the National Academy of Sciences. 3: 73–83. http://psychclassics.yorku.ca/Peirce/small-diffs.htm
- Hacking, Ian (September 1988). "Telepathy: Origins of Randomization in Experimental Design". Isis. 79 (3): 427–451. doi:10.1086/354775. JSTOR 234674. MR 1013489.
- Stephen M. Stigler (November 1992). "A Historical View of Statistical Concepts in Psychology and Educational Research". American Journal of Education. 101 (1): 60–70. doi:10.1086/444032.
- Trudy Dehue (December 1997). "Deception, Efficiency, and Random Groups: Psychology and the Gradual Origination of the Random Group Design" (PDF). Isis. 88 (4): 653–673. doi:10.1086/383850. PMID 9519574.
- Anglemyer A, Horvath HT, Bero L (April 2014). "Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials". Cochrane Database Syst Rev. 4 (4): MR000034. doi:10.1002/14651858.MR000034.pub2. PMID 24782322.
- Odgaard-Jensen J, Vist G, et al. (April 2011). "Randomisation to protect against selection bias in healthcare trials". Cochrane Database Syst Rev (4): MR000012. doi:10.1002/14651858.MR000012.pub3. PMC 7150228. PMID 21491415.
- Howick J, Mebius A (2014). "In search of justification for the unpredictability paradox". Trials. 15: 480. doi:10.1186/1745-6215-15-480. PMC 4295227. PMID 25490908.
- Caliński, Tadeusz & Kageyama, Sanpei (2000). Block designs: A Randomization approach, Volume I: Analysis. Lecture Notes in Statistics. 150. New York: Springer-Verlag. ISBN 978-0-387-98578-7.
- Caliński, Tadeusz & Kageyama, Sanpei (2003). Block designs: A Randomization approach, Volume II: Design. Lecture Notes in Statistics. 170. New York: Springer-Verlag. ISBN 978-0-387-95470-7.
- Hacking, Ian (September 1988). "Telepathy: Origins of Randomization in Experimental Design". Isis. 79 (3): 427–451. doi:10.1086/354775. JSTOR 234674. MR 1013489.
- Hinkelmann, Klaus; Kempthorne, Oscar (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (Second ed.). Wiley. ISBN 978-0-471-72756-9. MR 2363107.
- Kempthorne, Oscar (1992). "Intervention experiments, randomization and inference". In Malay Ghosh and Pramod K. Pathak (ed.). Current Issues in Statistical Inference—Essays in Honor of D. Basu. Institute of Mathematical Statistics Lecture Notes - Monograph Series. Hayward, CA: Institute for Mathematical Statistics. pp. 13–31. doi:10.1214/lnms/1215458836. ISBN 978-0-940600-24-9. MR 1194407.
