Scandium fluoride
Scandium(III) fluoride, ScF3, is an ionic compound. It is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Scandium(III) fluoride | |
| Other names
Scandium trifluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.033.854 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ScF3 | |
| Molar mass | 101.95112 g/mol |
| Appearance | bright white powder |
| Density | 2.53 g/cm3 |
| Melting point | 1,552 °C (2,826 °F; 1,825 K)[1] |
| Boiling point | 1,607 °C (2,925 °F; 1,880 K)[1] |
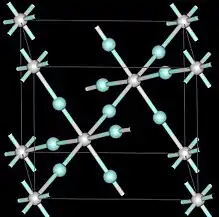
| Structure | |
| Cubic, Pm3m | |
| Pm3m, No. 221 | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Scandium(III) chloride Scandium(III) bromide Scandium(III) iodide |
Other cations |
Yttrium(III) fluoride |
Related compounds |
Scandium(III) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
ScF3 can be produced by reacting scandium and fluorine.[2] It is also formed during the extraction from the ore thortveitite by the reaction of Sc2O3 with ammonium bifluoride at high temperature:[3]
- Sc2O3 + 6 NH4HF2 → 2 ScF3 + 6 NH4F + 3 H2O
The resulting mixture contains a number of metal fluorides and this is reduced by reaction with calcium metal at high temperature.[3] Further purification steps are required to produce usable metallic scandium.[3]
Properties
Scandium trifluoride exhibits the unusual property of negative thermal expansion, meaning it shrinks when heated. This phenomenon is explained by the quartic oscillation of the fluoride ions. The energy stored in the bending strain of the fluoride ion is proportional to the fourth power of the displacement angle, unlike most other materials where it is proportional to the square of the displacement. A fluorine atom is bound to two scandium atoms, and as temperature increases the fluorine oscillates more perpendicularly to its bonds. This motion draws the scandium atoms together throughout the bulk material, which contracts.[4] ScF3 exhibits this property from at least 10 K to 1100 K above which it shows the normal positive thermal expansion; furthermore, the material has cubic symmetry over this entire temperature range, and up to at least 1600 K at ambient pressure. The negative thermal expansion at very low temperatures is quite strong (coefficient of thermal expansion around -14 ppm/K between 60 and 110 K).[5]
At ambient pressures scandium trifluoride adopts the cubic crystal system, using the perovskite structure with one metal position vacant.[6] The unit cell dimension is 4.01 Å.[6] Under pressure scandium trifluoride also forms different crystal structures with rhombohedral, and above 3 GPa tetrahedral.[6]
References
- Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier, ISBN 0-12-352651-5.
- S.A.Cotton, Scandium, Yttrium and the Lanthanides: Inorganic and Coordination Chemistry, Encyclopedia of Inorganic Chemistry, 1994, John Wiley & Sons, ISBN 0-471-93620-0.
- Pradyot Patnaik, 2003, Handbook of Inorganic Chemicals, McGraw-Hill Professional, ISBN 0-07-049439-8.
- Woo, Marcus (7 November 2011). "An incredible shrinking material: Engineers reveal how scandium trifluoride contracts with heat". Physorg. Retrieved 8 November 2011.
- Greve, Benjamin K.; Kenneth L. Martin; Peter L. Lee; Peter J. Chupas; Karena W. Chapman; Angus P. Wilkinson (19 October 2010). "Pronounced Negative Thermal Expansion from a Simple Structure: Cubic ScF3". Journal of the American Chemical Society. 132 (44): 15496–15498. doi:10.1021/ja106711v. PMID 20958035.
- Aleksandrov, K. S.; V. N. Voronov; A. N. Vtyurin; A. S. Krylov; M. S. Molokeev; M. S. Pavlovskiĭ; S. V. Goryaĭnov; A. Yu. Likhacheva; A. I. Ancharov (2009). "Pressure-induced phase transition in the cubic ScF3 crystal". Physics of the Solid State. 51 (4): 810–816. doi:10.1134/S1063783409040295. ISSN 1063-7834. S2CID 119874020.
