COPII
COPII is a coatomer, a type of vesicle coat protein that transports proteins from the rough endoplasmic reticulum to the Golgi apparatus.[2][3] This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI protein. The name "COPII" refers to the specific coat protein complex that initiates the budding process. The coat consists of large protein subcomplexes that are made of four different protein subunits.
| Sec23 homolog A | |||||||
|---|---|---|---|---|---|---|---|
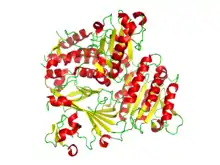 Ribbon diagram of the crystallographic structure of the COPII heterodimer of Sec23 and Sec24. Alpha helices are in red and the beta sheets are in yellow.[1] | |||||||
| Identifiers | |||||||
| Symbol | SEC23A | ||||||
| NCBI gene | 856311 | ||||||
| HGNC | 10701 | ||||||
| OMIM | 610511 | ||||||
| PDB | 1M2V | ||||||
| RefSeq | NM_006364 | ||||||
| UniProt | Q15436 | ||||||
| Other data | |||||||
| Locus | Chr. 14 q21.1 | ||||||
| |||||||
| SEC24 family, member A | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | SEC24A | ||||||
| NCBI gene | 10802 | ||||||
| HGNC | 10703 | ||||||
| OMIM | 607183 | ||||||
| PDB | 1M2V | ||||||
| RefSeq | XM_001132082 | ||||||
| UniProt | O95486 | ||||||
| Other data | |||||||
| Locus | Chr. 5 q31.1 | ||||||
| |||||||
Coat proteins
COPII's coat is composed of five proteins: Sar1, Sec23, Sec24, Sec13, and Sec31.[4] These proteins dimerize to form larger protein complexes:
It is important to note that there are five different types of proteins that constitute the COPII coat, but multiple proteins of the same variety compose the protein complexes critical to the formation of the COPII coat.
These coat proteins are necessary but insufficient to direct or dock the vesicle to the correct target membrane. SNARE, cargo, and other proteins are also needed for these processes to occur.
Budding process
Assembly of COPII vesicles can be summarized as:
- Sar1-GDP interacts with the ER transmembrane protein Sec12.
- Sar1-GTP recruits Sec23/Sec24 coat protein to form a pre-budding complex.
- Pre-budding complex (composed of Sar1-GTP bound with Sec23/24) recruits Sec13/Sec31, which forms the second coat layer.
- Sec13/Sec31 complex forms a cage-like outer coat (similar to the formation of clathrin vesicles).
Sar1p is a GTPase that acts as a "switch" that flips between an activated membrane embedded GTP-bound form, and an inactive soluble GDP-bound form.[5] Inactive GDP-bound Sar1p is attracted to the cytosolic side of the endoplasmic reticulum.
Sec12, a transmembrane protein found in the ER acts as a Guanine nucleotide exchange factor by stimulating the release of GDP to allow the binding of GTP in Sar1.
GTP-bound Sar1p undergoes a conformational change which exposes an N-terminal amphipathic a-helix (other sources say a hydrophobic tail) to be inserted into the ER membrane. Membrane-bound Sar1p recruits the Sec23p/24p complex to form what is collectively known as the pre-budding complex. Sec23/Sec24 specifically binds to specific sorting signals in membrane cargo protein cytosolic domains, these sorting signals do not share a simple signal motif like KDEL or KKXX. Recent research suggests that multiple ER export signals cooperate to segregate and exclude unassembled cargo.[4]
Pre-budding complex (composed of Sar1-GTP and Sec23/24) recruits the flexible Sec13p/31p complex, characterized by polymerization of the Sec13/31 complex with other Sec13/31 complexes to form a cuboctahedron with a broader lattice than its Clathrin vesicle analog. The formation of the cuboctahedron deforms the ER membrane and detaches the COPII vesicle (alongside cargo proteins and v-SNAREs), completing the COPII vesicle budding process.[6]
Some proteins are found to be responsible for selectively packaging cargos into COPII vesicles. More recent research suggests th Sec23/Sec24-Sar1 complex participates in cargo selection.[6] For example, Erv29p in Saccharomyces cerevisiae is found to be necessary for packaging glycosylated pro-α-factor.[7]
After the COPII vesicle forms, the COPII coat proteins remain assembled to allow the Sec23/Sec24 complex to interact with a tethering factor on the Cis-Golgi membrane. When the COPII vesicle is in close proximity to the Cis-Golgi membrane, it sheds its coat and the components are recycled to function for another vesicle.
Conformational changes
CopII has three specific binding sites that can each be complexed. The adjacent picture (Sed5) uses the Sec22 t-SNARE complex to bind. This site is more strongly bound, and therefore is more favored. (Embo)
References
- PDB: 3EH1; Mancias JD, Goldberg J (November 2008). "Structural basis of cargo membrane protein discrimination by the human COPII coat machinery". EMBO J. 27 (21): 2918–28. doi:10.1038/emboj.2008.208. PMC 2580787. PMID 18843296.
- Lee MC, Miller EA (August 2007). "Molecular mechanisms of COPII vesicle formation". Semin. Cell Dev. Biol. 18 (4): 424–34. doi:10.1016/j.semcdb.2007.06.007. PMID 17686639.
- Hughes H, Stephens DJ (February 2008). "Assembly, organization, and function of the COPII coat". Histochem. Cell Biol. 129 (2): 129–51. doi:10.1007/s00418-007-0363-x. PMC 2228377. PMID 18060556.
- D'Arcangelo, Jennifer G.; Stahmer, Kyle R.; Miller, Elizabeth A. (November 2013). "Vesicle-mediated export from the ER: COPII coat function and regulation". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833 (11): 2464–2472. doi:10.1016/j.bbamcr.2013.02.003. PMC 3676692. PMID 23419775.
- Bonifacino JS, Glick BS (January 2004). "The mechanisms of vesicle budding and fusion". Cell. 116 (2): 153–66. doi:10.1016/s0092-8674(03)01079-1. PMID 14744428. S2CID 1777139.
- Fath S, Mancias JD, Bi X, Goldberg J (June 2007). "Structure and organization of coat proteins in the COPII cage". Cell. 129 (7): 1325–36. doi:10.1016/j.cell.2007.05.036. PMID 17604721. S2CID 10692166.
- Belden WJ, Barlowe C (November 2001). "Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles". Science. 294 (5546): 1528–31. Bibcode:2001Sci...294.1528B. doi:10.1126/science.1065224. PMID 11711675. S2CID 29870942.
- 1PCX; 1PD0; Mossessova E, Bickford LC, Goldberg J (August 2003). "SNARE selectivity of the COPII coat". Cell. 114 (4): 483–95. doi:10.1016/S0092-8674(03)00608-1. PMID 12941276. S2CID 11379372.