Insect morphology
Insect morphology is the study and description of the physical form of insects. The terminology used to describe insects is similar to that used for other arthropods due to their shared evolutionary history. Three physical features separate insects from other arthropods: they have a body divided into three regions (head, thorax, and abdomen), have three pairs of legs, and mouthparts located outside of the head capsule. It is this position of the mouthparts which divides them from their closest relatives, the non-insect hexapods, which includes Protura, Diplura, and Collembola.
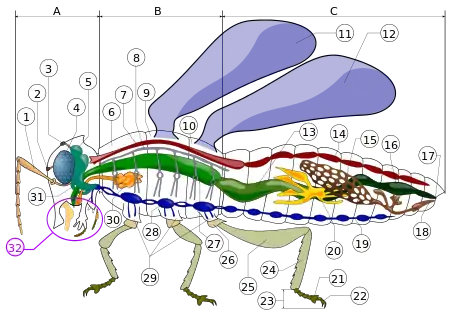
Legend of body parts
Tagmata: A - Head, B - Thorax, C - Abdomen.
- antenna
- ocelli (lower)
- ocelli (upper)
- compound eye
- brain (cerebral ganglia)
- prothorax
- dorsal blood vessel
- tracheal tubes (trunk with spiracle)
- mesothorax
- metathorax
- forewing
- hindwing
- mid-gut (stomach)
- dorsal tube (heart)
- ovary
- hind-gut (intestine, rectum & anus)
- anus
- oviduct
- nerve cord (abdominal ganglia)
- Malpighian tubes
- tarsal pads
- claws
- tarsus
- tibia
- femur
- trochanter
- fore-gut (crop, gizzard)
- thoracic ganglion
- coxa
- salivary gland
- subesophageal ganglion
- mouthparts
There is enormous variation in body structure amongst insect species. Individuals can range from 0.3 mm (fairyflies) to 30 cm across (great owlet moth);[1]:7 have no eyes or many; well-developed wings or none; and legs modified for running, jumping, swimming, or even digging. These modifications allow insects to occupy almost every ecological niche on the planet, except the deep ocean. This article describes the basic insect body and some of the major variations of the different body parts; in the process it defines many of the technical terms used to describe insect bodies.
Anatomy summary
Insects, like all arthropods, have no interior skeleton; instead, they have an exoskeleton, a hard outer layer made mostly of chitin which protects and supports the body. The insect body is divided into three parts: the head, thorax, and abdomen.[2] The head is specialized for sensory input and food intake; the thorax, which is the anchor point for the legs and wings (if present), is specialized for locomotion; and the abdomen for digestion, respiration, excretion, and reproduction.[1]:22–48 Although the general function of the three body regions is the same across all insect species, there are major differences in basic structure, with wings, legs, antennae, and mouthparts being highly variable from group to group.[3]
External
Exoskeleton
The insect outer skeleton, the cuticle, is made up of two layers; the epicuticle, which is a thin, waxy, water-resistant outer layer and contains no chitin, and the layer under it called the procuticle. This is chitinous and much thicker than the epicuticle and has two layers, the outer is the exocuticle while the inner is the endocuticle. The tough and flexible endocuticle is built from numerous layers of fibrous chitin and proteins, criss-crossing each other in a sandwich pattern, while the exocuticle is rigid and sclerotized.[1]:22–24 The exocuticle is greatly reduced in many soft-bodied insects, especially the larval stages (e.g., caterpillars). Chemically, chitin is a long-chain polymer of a N-acetylglucosamine, a derivative of glucose. In its unmodified form, chitin is translucent, pliable, resilient and quite tough. In arthropods, however, it is often modified, becoming embedded in a hardened proteinaceous matrix, which forms much of the exoskeleton. In its pure form, it is leathery, but when encrusted in calcium carbonate, it becomes much harder.[4] The difference between the unmodified and modified forms can be seen by comparing the body wall of a caterpillar (unmodified) to a beetle (modified).
From the embryonic stages itself, a layer of columnar or cuboidal epithelial cells gives rise to the external cuticle and an internal basement membrane. The majority of insect material is held in the endocuticle. The cuticle provides muscular support and acts as a protective shield as the insect develops. However, since it cannot grow, the external sclerotised part of the cuticle is periodically shed in a process called "moulting". As the time for moulting approaches, most of the exocuticle material is reabsorbed. In moulting, first the old cuticle separates from the epidermis (apolysis). Enzymatic moulting fluid is released between the old cuticle and epidermis, which separates the exocuticle by digesting the endocuticle and sequestering its material for the new cuticle. When the new cuticle has formed sufficiently, the epicuticle and reduced exocuticle are shed in ecdysis.[5]:16–20
The four principal regions of an insect body segment are: tergum or dorsal, sternum or ventral and the two pleura or laterals. Hardened plates in the exoskeleton are called sclerites, which are subdivisions of the major regions - tergites, sternites and pleurites, for the respective regions tergum, sternum, and pleuron.[6]
Head



The head in most insects is enclosed in a hard, heavily sclerotized, exoskeletal head capsule'. The main exception is in those species whose larvae are not fully sclerotised, mainly some holometabola; but even most unsclerotised or weakly sclerotised larvae tend to have well sclerotised head capsules, for example the larvae of Coleoptera and Hymenoptera. The larvae of Cyclorrhapha however, tend to have hardly any head capsule at all.
The head capsule bears most of the main sensory organs, including the antennae, ocelli, and the compound eyes. It also bears the mouthparts. In the adult insect the head capsule is apparently unsegmented, though embryological studies show it to consist of six segments that bear the paired head appendages, including the mouthparts, each pair on a specific segment.[7] Each such pair occupies one segment, though not all segments in modern insects bear any visible appendages.
Of all the insect orders, Orthoptera most conveniently display the greatest variety of features found in the heads of insects, including the sutures and sclerites.[6] Here, the vertex, or the apex (dorsal region), is situated between the compound eyes for insects with hypognathous and opisthognathous heads. In prognathous insects, the vertex is not found between the compound eyes, but rather, where the ocelli are normally found. This is because the primary axis of the head is rotated 90° to become parallel to the primary axis of the body. In some species, this region is modified and assumes a different name.[8]:13
The ecdysial suture is made of the coronal, frontal, and epicranial sutures plus the ecdysial and cleavage lines, which vary among different species of insects. The ecdysial suture is longitudinally placed on the vertex and separates the epicranial halves of the head to the left and right sides. Depending on the insect, the suture may come in different shapes: like either a Y, U, or V. Those diverging lines that make up the ecdysial suture are called the frontal or frontogenal sutures. Not all species of insects have frontal sutures, but in those that do, the sutures split open during ecdysis, which helps provide an opening for the new instar to emerge from the integument.
The frons is that part of the head capsule that lies ventrad or anteriad of the vertex. The frons varies in size relative to the insect, and in many species the definition of its borders is arbitrary, even in some insect taxa that have well-defined head capsules. In most species, though, the frons is bordered at its anterior by the frontoclypeal or epistomal sulcus above the clypeus. Laterally it is limited by the fronto-genal sulcus, if present, and the boundary with the vertex, by the ecdysial cleavage line, if it is visible. If there is a median ocellus, it generally is on the frons, though in some insects such as many Hymenoptera, all three ocelli appear on the vertex. A more formal definition is that it is the sclerite from which the pharyngeal dilator muscles arise, but in many contexts that too, is not helpful.[7] In the anatomy of some taxa, such as many Cicadomorpha, the front of the head is fairly clearly distinguished and tends to be broad and sub-vertical; that median area commonly is taken to be the frons.[9]
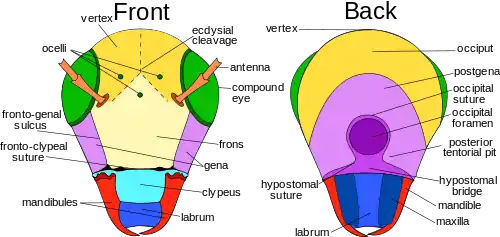
The clypeus is a sclerite between the face and labrum, which is dorsally separated from the frons by the frontoclypeal suture in primitive insects. The clypeogenal suture laterally demarcates the clypeus, with the clypeus ventrally separated from the labrum by the clypeolabral suture. The clypeus differs in shape and size, such as species of Lepidoptera with a large clypeus with elongated mouthparts. The cheek or gena forms the sclerotized area on each side of the head below the compound eyes extending to the gular suture. Like many of the other parts making up the insect's head, the gena varies among species, with its boundaries difficult to establish. For example, in dragonflies and damselflies, it is between the compound eyes, clypeus, and mouthparts. The postgena is the area immediately posteriad, or posterior or lower on the gena of pterygote insects, and forms the lateral and ventral parts of the occipital arch. The occipital arch is a narrow band forming the posterior edge of the head capsule arching dorsally over the foramen. The subgenal area is usually narrow, located above the mouthparts; this area also includes the hypostoma and pleurostoma.[8]:13–14 The vertex extends anteriorly above the bases of the antennae as a prominent, pointed, concave rostrum. The posterior wall of the head capsule is penetrated by a large aperture, the foramen. Through it pass the organ systems, such as nerve cord, esophagus, salivary ducts, and musculature, connecting the head with the thorax.[10]
On the posterior aspect of the head are the occiput, postgena, occipital foramen, posterior tentorial pit, gula, postgenal bridge, hypostomal suture and bridge, and the mandibles, labium, and maxilla. The occipital suture is well founded in species of Orthoptera, but not so much in other orders. Where found, the occipital suture is the arched, horseshoe-shaped groove on the back of the head, ending at the posterior of each mandible. The postoccipital suture is a landmark on the posterior surface of the head, and is typically near the occipital foremen. In pterygotes, the postocciput forms the extreme posterior, often U-shaped, which forms the rim of the head extending to the postoccipital suture. In pterygotes, such as those of Orthoptera, the occipital foramen and the mouth are not separated. The three types of occipital closures, or points under the occipital foramen that separate the two lower halves of the postgena, are: the hypostomal bridge, the postgenal bridge, and the gula. The hypostomal bridge is usually found in insects with hypognathous orientation. The postgenal bridge is found in the adults of species of higher Diptera and aculeate Hymenoptera, while the gula is found on some Coleoptera, Neuroptera, and Isoptera, which typically display prognathous-oriented mouthparts.[8]:15
Compound eyes and ocelli
Most insects have one pair of large, prominent compound eyes composed of units called ommatidia (ommatidium, singular), possibly up to 30,000 in a single compound eye of, for example, large dragonflies. This type of eye gives less resolution than eyes found in vertebrates, but it gives acute perception of movement and usually possesses UV- and green sensitivity and may have additional sensitivity peaks in other regions of the visual spectrum. Often an ability to detect the E-vector of polarized light exists polarization of light.[11] There can also be an additional two or three ocelli, which help detect low light or small changes in light intensity. The image perceived is a combination of inputs from the numerous ommatidia, located on a convex surface, thus pointing in slightly different directions. Compared with simple eyes, compound eyes possess very large view angles and better acuity than the insect's dorsal ocelli, but some stemmatal (= larval eyes), for example those of sawfly larvae (Tenthredinidae) with an acuity 4 degrees and very high polarization sensitivity, match the performance of compound eyes.[12] [13]

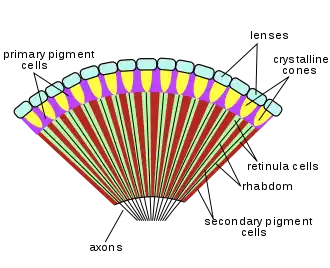
Because the individual lenses are so small, the effects of diffraction impose a limit on the possible resolution that can be obtained (assuming they do not function as phased arrays). This can only be countered by increasing lens size and number. To see with a resolution comparable to our simple eyes, humans would require compound eyes that would each reach the size of their heads. Compound eyes fall into two groups: apposition eyes, which form multiple inverted images, and superposition eyes, which form a single erect image.[14][15] Compound eyes grow at their margins by the addition of new ommatidia.[16]
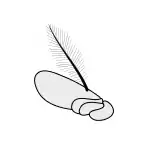
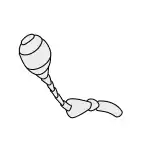
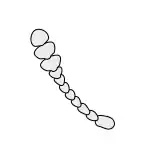
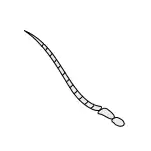
Antennae

Antennae, sometimes called "feelers", are flexible appendages located on the insect's head which are used for sensing the environment. Insects are able to feel with their antennae because of the fine hairs (setae) that cover them.[17]:8–11 However, touch is not the only thing that antennae can detect; numerous tiny sensory structures on the antennae allow insects to sense smells, temperature, humidity, pressure, and even potentially sense themselves in space.[17]:8–11[18][19] Some insects, including bees and some groups of flies can also detect sound with their antennae.[20]
The number of segments in an antenna varies considerably amongst insects, with higher flies having only 3-6 segments,[21] while adult cockroaches can have over 140.[22] The general shape of the antennae is also quite variable, but the first segment (the one attached to the head) is always called the scape, and the second segment is called the pedicel. The remaining antennal segments or flagellomeres are called the flagellum.[17]:8–11
General insect antenna types are shown below:
 Aristate |
 Capitate |
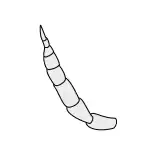
 Clavate |
 Filiform |
 Flabellate |
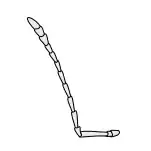 Geniculate |
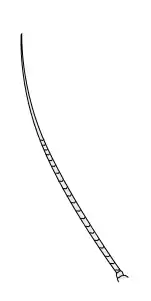 Setaceous |
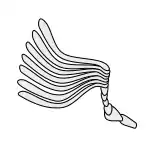 Lamellate |
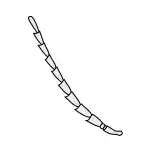
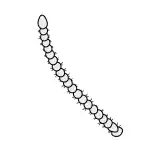 Moniliform |
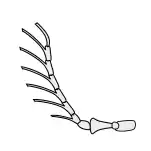 Pectinate |
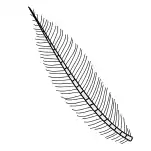 Plumose |
 Serrate |
 Stylate |
Mouthparts
The insect mouthparts consist of the maxilla, labium, and in some species, the mandibles.[8]:16[23] The labrum is a simple, fused sclerite, often called the upper lip, and moves longitudinally, which is hinged to the clypeus. The mandibles (jaws) are a highly sclerotized pair of structures that move at right angles to the body, used for biting, chewing, and severing food. The maxillae are paired structures that can also move at right angles to the body and possess segmented palps. The labium (lower lip) is the fused structure that moves longitudinally and possesses a pair of segmented palps.[24]
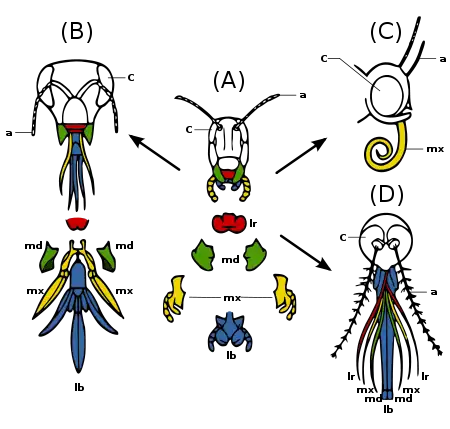
Legend: a - antennae
c - compound eye
lb - labium
lr - labrum
md - mandibles
mx - maxillae
The mouthparts, along with the rest of the head, can be articulated in at least three different positions: prognathous, opisthognathous, and hypognathous. In species with prognathous articulation, the head is positioned vertically aligned with the body, such as species of Formicidae; while in a hypognathous type, the head is aligned horizontally adjacent to the body. An opisthognathous head is positioned diagonally, such as species of Blattodea and some Coleoptera.[25] The mouthparts vary greatly between insects of different orders, but the two main functional groups are mandibulate and haustellate. Haustellate mouthparts are used for sucking liquids and can be further classified by the presence of stylets, which include piercing-sucking, sponging, and siphoning. The stylets are needle-like projections used to penetrate plant and animal tissues. The stylets and the feeding tube form the modified mandibles, maxilla, and hypopharynx.[24]
- Mandibulate mouthparts, among the most common in insects, are used for biting and grinding solid foods.
- Piercing-sucking mouthparts have stylets, and are used to penetrate solid tissue and then suck up liquid food.
- Sponging mouthparts are used to sponge and suck liquids, and lack stylets (e.g. most Diptera).
- Siphoning mouthparts lack stylets and are used to suck liquids, and are commonly found among species of Lepidoptera.
Mandibular mouthparts are found in species of Odonata, adult Neuroptera, Coleoptera, Hymenoptera, Blattodea, Orthoptera, and Lepidoptera. However, most adult Lepidoptera have siphoning mouthparts, while their larvae (commonly called caterpillars) have mandibles.
Mandibulate
The labrum is a broad lobe forming the roof of the preoral cavity, suspended from the clypeus in front of the mouth and forming the upper lip.[1]:22–24 On its inner side, it is membranous and may be produced into a median lobe, the epipharynx, bearing some sensilla. The labrum is raised away from the mandibles by two muscles arising in the head and inserted medially into the anterior margin of the labrum. It is closed against the mandibles in part by two muscles arising in the head and inserted on the posterior lateral margins on two small sclerites, the tormae, and, at least in some insects, by a resilin spring in the cuticle at the junction of the labrum with the clypeus. [26] Until recently, the labrum generally was considered to be associated with first head segment. However, recent studies of the embryology, gene expression, and nerve supply to the labrum show it is innervated by the tritocerebrum of the brain, which is the fused ganglia of the third head segment. This is formed from fusion of parts of a pair of ancestral appendages found on the third head segment, showing their relationship.[1]:22–24 Its ventral, or inner, surface is usually membranous and forms the lobe-like epipharynx, which bears mechanosensilla and chemosensilla.[27][28]
Chewing insects have two mandibles, one on each side of the head. The mandibles are positioned between the labrum and maxillae. The mandibles cut and crush food, and may be used for defense; generally, they have an apical cutting edge, and the more basal molar area grinds the food. They can be extremely hard (around 3 on Mohs, or an indentation hardness of about 30 kg/mm2); thus, many termites and beetles have no physical difficulty in boring through foils made from such common metals as copper, lead, tin, and zinc.[1]:22–24 The cutting edges are typically strengthened by the addition of zinc, manganese, or rarely, iron, in amounts up to about 4% of the dry weight.[27] They are typically the largest mouthparts of chewing insects, being used to masticate (cut, tear, crush, chew) food items. They open outwards (to the sides of the head) and come together medially. In carnivorous, chewing insects, the mandibles can be modified to be more knife-like, whereas in herbivorous chewing insects, they are more typically broad and flat on their opposing faces (e.g., caterpillars). In male stag beetles, the mandibles are modified to such an extent as to not serve any feeding function, but are instead are used to defend mating sites from other males. In ants, the mandibles also serve a defensive function (particularly in soldier castes). In bull ants, the mandibles are elongated and toothed, used as hunting (and defensive) appendages.
Situated beneath the mandibles, paired maxillae manipulate food during mastication. Maxillae can have hairs and "teeth" along their inner margins. At the outer margin, the galea is a cupped or scoop-like structure, which sits over the outer edge of the labium. They also have palps, which are used to sense the characteristics of potential foods. The maxillae occupy a lateral position, one on each side of the head behind the mandibles. The proximal part of the maxilla consists of a basal cardo, which has a single articulation with the head, and a flat plate, the stipes, hinged to the cardo. Both cardo and stipes are loosely joined to the head by membrane so they are capable of movement. Distally on the stipes are two lobes, an inner lacinea and an outer galea, one or both of which may be absent. More laterally on the stipes is a jointed, leglike palp made up of a number of segments; in Orthoptera there are five. Anterior and posterior rotator muscles are inserted on the cardo, and ventral adductor muscles arising on the tentorium are inserted on both cardo and stipes. Arising in the stipes are flexor muscles of lacinea and galea and another lacineal flexor arises in the cranium, but neither the lacinea nor the galea has an extensor muscle. The palp has levator and depressor muscles arising in the stipes, and each segment of the palp has a single muscle causing flexion of the next segment.[26]
In mandibulate mouthparts, the labium is a quadrupedal structure, although it is formed from two fused secondary maxillae. It can be described as the floor of the mouth. With the maxillae, it assists with manipulation of food during mastication or chewing or, in the unusual case of the dragonfly nymph, extends out to snatch prey back to the head, where the mandibles can eat it. The labium is similar in structure to the maxilla, but with the appendages of the two sides fused by the midline, so they come to form a median plate. The basal part of the labium, equivalent to the maxillary cardines and possibly including a part of the sternum of the labial segment, is called the postmentum. This may be subdivided into a proximal submentum and a distal mentum. Distal to the postmentum, and equivalent to the fused maxillary stipites, is the prementum. The prementum closes the preoral cavity from behind. Terminally, it bears four lobes, two inner glossae, and two outer paraglossae, which are collectively known as the ligula. One or both pairs of lobes may be absent or they may be fused to form a single median process. A palp arises from each side of the prementum, often being three-segmented.[26]
The hypopharynx is a median lobe immediately behind the mouth, projecting forwards from the back of the preoral cavity; it is a lobe of uncertain origin, but perhaps associated with the mandibular segment;[26] in apterygotes, earwigs, and nymphal mayflies, the hypopharynx bears a pair of lateral lobes, the superlinguae (singular: superlingua). It divides the cavity into a dorsal food pouch, or cibarium, and a ventral salivarium into which the salivary duct opens.[1]:22–24 It is commonly found fused to the libium.[27] Most of the hypopharynx is membranous, but the adoral face is sclerotized distally, and proximally contains a pair of suspensory sclerites extending upwards to end in the lateral wall of the stomodeum. Muscles arising on the frons are inserted into these sclerites, which distally are hinged to a pair of lingual sclerites. These, in turn, have inserted into them antagonistic pairs of muscles arising on the tentorium and labium. The various muscles serve to swing the hypopharynx forwards and back, and in the cockroach, two more muscles run across the hypopharynx and dilate the salivary orifice and expand the salivarium.[26]
- Examples of mandibles
Piercing-sucking
Mouthparts can have multiple functions. Some insects combine piercing parts along with sponging ones which are then used to pierce through tissues of plants and animals. Female mosquitoes feed on blood (hemophagous) making them disease vectors. The mosquito mouthparts consist of the proboscis, paired mandibles and maxillae. The maxillae form needle-like structures, called stylets, which are enclosed by the labium. When mosquito bites, maxillae penetrate the skin and anchor the mouthparts, thus allowing other parts to be inserted. The sheath-like labium slides back, and the remaining mouthparts pass through its tip and into the tissue. Then, through the hypopharynx, the mosquito injects saliva, which contains anticoagulants to stop the blood from clotting. And finally, the labrum (upper lip) is used to suck up the blood. Species of the genus Anopheles are characterized by their long palpi (two parts with widening end), almost reaching the end of labrum.[29]
- Examples of piercing mouthparts




_(1).jpg.webp) Horsefly (female)
Horsefly (female)
Siphoning
The proboscis is formed from maxillary galeae and is adaption found in some insects for sucking.[30] The muscles of the cibarium or pharynx are strongly developed and form the pump. In Hemiptera and many Diptera, which feed on fluids within plants or animals, some components of the mouthparts are modified for piercing, and the elongated structures are called stylets. The combined tubular structures are referred to as the proboscis, although specialized terminology is used in some groups.
In species of Lepidoptera, it consists of two tubes held together by hooks and separable for cleaning. Each tube is inwardly concave, thus forming a central tube through which moisture is sucked. Suction is effected through the contraction and expansion of a sac in the head.[31] The proboscis is coiled under the head when the insect is at rest, and is extended only when feeding.[30] The maxillary palpi are reduced or even vestigial.[32] They are conspicuous and five-segmented in some of the more basal families, and are often folded.[8] The shape and dimensions of the proboscis have evolved to give different species wider and therefore more advantageous diets.[30] There is an allometric scaling relationship between body mass of Lepidoptera and length of proboscis[33] from which an interesting adaptive departure is the unusually long-tongued hawk moth Xanthopan morganii praedicta. Charles Darwin predicted the existence and proboscis length of this moth before its discovery based on his knowledge of the long-spurred Madagascan star orchid Angraecum sesquipedale.[34]
- Examples of siphoning mouthparts
Sponging
The mouthparts of insects that feed on fluids are modified in various ways to form a tube through which liquid can be drawn into the mouth and usually another through which saliva passes. The muscles of the cibarium or pharynx are strongly developed to form a pump.[26] In nonbiting flies, the mandibles are absent and other structures are reduced; the labial palps have become modified to form the labellum, and the maxillary palps are present, although sometimes short. In Brachycera, the labellum is especially prominent and used for sponging liquid or semiliquid food.[35] The labella are a complex structure consisting of many grooves, called pseudotracheae, which sop up liquids. Salivary secretions from the labella assist in dissolving and collecting food particles so they can be more easily taken up by the pseudotracheae; this is thought to occur by capillary action. The liquid food is then drawn up from the pseudotracheae through the food channel into the esophagus.[36]
The mouthparts of bees are of a chewing and lapping-sucking type. Lapping is a mode of feeding in which liquid or semiliquid food adhering to a protrusible organ, or "tongue", is transferred from substrate to mouth. In the honey bee (Hymenoptera: Apidae: Apis mellifera), the elongated and fused labial glossae form a hairy tongue, which is surrounded by the maxillary galeae and the labial palps to form a tubular proboscis containing a food canal. In feeding, the tongue is dipped into the nectar or honey, which adheres to the hairs, and then is retracted so the adhering liquid is carried into the space between the galeae and labial palps. This back-and-forth glossal movement occurs repeatedly. Movement of liquid to the mouth apparently results from the action of the cibarial pump, facilitated by each retraction of the tongue pushing liquid up the food canal.[1]:22–24
- Examples of sponging mouthparts
Thorax
The insect thorax has three segments: the prothorax, mesothorax, and metathorax. The anterior segment, closest to the head, is the prothorax; its major features are the first pair of legs and the pronotum. The middle segment is the mesothorax; its major features are the second pair of legs and the anterior wings, if any. The third, the posterior, thoracic segment, abutting the abdomen, is the metathorax, which bears the third pair of legs and the posterior wings. Each segment is delineated by an intersegmental suture. Each segment has four basic regions. The dorsal surface is called the tergum (or notum, to distinguish it from the abdominal terga).[1]:22–24 The two lateral regions are called the pleura (singular: pleuron), and the ventral aspect is called the sternum. In turn, the notum of the prothorax is called the pronotum, the notum for the mesothorax is called the mesonotum and the notum for the metathorax is called the metanotum. Continuing with this logic, there is also the mesopleura and metapleura, as well as the mesosternum and metasternum.[8]
The tergal plates of the thorax are simple structures in apterygotes and in many immature insects, but are variously modified in winged adults. The pterothoracic nota each have two main divisions: the anterior, wing-bearing alinotum and the posterior, phragma-bearing postnotum. Phragmata (singular: phragma) are plate-like apodemes that extend inwards below the antecostal sutures, marking the primary intersegmental folds between segments; phragmata provide attachment for the longitudinal flight muscles. Each alinotum (sometimes confusingly referred to as a "notum") may be traversed by sutures that mark the position of internal strengthening ridges, and commonly divides the plate into three areas: the anterior prescutum, the scutum, and the smaller posterior scutellum. The lateral pleural sclerites are believed to be derived from the subcoxal segment of the ancestral insect leg. These sclerites may be separate, as in silverfish, or fused into an almost continuous sclerotic area, as in most winged insects.[1]:22–24
Prothorax
The pronotum of the prothorax may be simple in structure and small in comparison with the other nota, but in beetles, mantids, many bugs, and some Orthoptera, the pronotum is expanded, and in cockroaches, it forms a shield that covers part of the head and mesothorax.[8][1]:22–24
Pterothorax
Because the mesothorax and metathorax hold the wings, they have a combined name called the pterothorax (pteron = wing). The forewing, which goes by different names in different orders (e.g., the tegmina in Orthoptera and elytra in Coleoptera), arises between the mesonotum and the mesopleuron, and the hindwing articulates between the metanotum and metapleuron. The legs arise from the mesopleuron and metapleura. The mesothorax and metathorax each have a pleural suture (mesopleural and metapleural sutures) that runs from the wing base to the coxa of the leg. The sclerite anterior to the pleural suture is called the episternum (serially, the mesepisternum and metepisternum). The sclerite posterior to the suture is called the epimiron (serially, the mesepimiron and metepimiron). Spiracles, the external organs of the respiratory system, are found on the pterothorax, usually one between the pro- and mesopleoron, as well as one between the meso- and metapleuron.[8]
The ventral view or sternum follows the same convention, with the prosternum under the prothorax, the mesosternum under the mesothorax and the metasternum under the metathorax. The notum, pleura, and sternum of each segment have a variety of different sclerites and sutures, varying greatly from order to order, and they will not be discussed in detail in this section.[8]
Wings
Most phylogenetically advanced insects have two pairs of wings located on the second and third thoracic segments.[1]:22–24 Insects are the only invertebrates to have developed flight capability, and this has played an important part in their success. Insect flight is not very well understood, relying heavily on turbulent aerodynamic effects. The primitive insect groups use muscles that act directly on the wing structure. The more advanced groups making up the Neoptera have foldable wings, and their muscles act on the thorax wall and power the wings indirectly.[1]:22–24 These muscles are able to contract multiple times for each single nerve impulse, allowing the wings to beat faster than would ordinarily be possible.
Insect flight can be extremely fast, maneuverable, and versatile, possibly due to the changing shape, extraordinary control, and variable motion of the insect wing. Insect orders use different flight mechanisms; for example, the flight of a butterfly can be explained using steady-state, nontransitory aerodynamics, and thin airfoil theory.
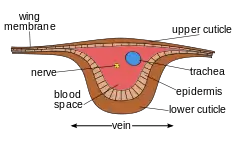
Internal
Each of the wings consists of a thin membrane supported by a system of veins. The membrane is formed by two layers of integument closely apposed, while the veins are formed where the two layers remain separate and the cuticle may be thicker and more heavily sclerotized. Within each of the major veins is a nerve and a trachea, and, since the cavities of the veins are connected with the hemocoel, hemolymph can flow into the wings.[26] As the wing develops, the dorsal and ventral integumental layers become closely apposed over most of their area, forming the wing membrane. The remaining areas form channels, the future veins, in which the nerves and tracheae may occur. The cuticle surrounding the veins becomes thickened and more heavily sclerotized to provide strength and rigidity to the wing. Hairs of two types may occur on the wings: microtrichia, which are small and irregularly scattered, and macrotrichia, which are larger, socketed, and may be restricted to veins. The scales of Lepidoptera and Trichoptera are highly modified macrotrichia.[27]
Veins
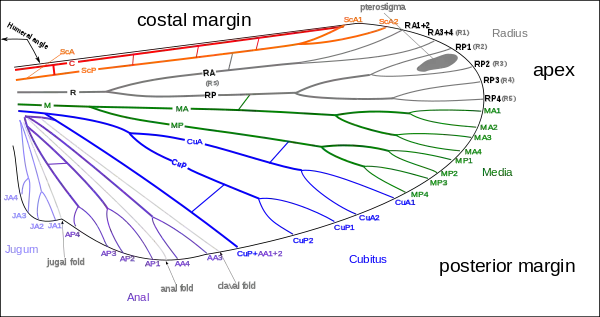
In some very small insects, the venation may be greatly reduced. In chalcid wasps, for instance, only the subcosta and part of the radius are present. Conversely, an increase in venation may occur by the branching of existing veins to produce accessory veins or by the development of additional, intercalary veins between the original ones, as in the wings of Orthoptera (grasshoppers and crickets). Large numbers of cross-veins are present in some insects, and they may form a reticulum as in the wings of Odonata (dragonflies and damselflies) and at the base of the forewings of Tettigonioidea and Acridoidea (katydids and grasshoppers, respectively).[26]
The archedictyon is the name given to a hypothetical scheme of wing venation proposed for the very first winged insect. It is based on a combination of speculation and fossil data. Since all winged insects are believed to have evolved from a common ancestor, the archediction represents the "template" that has been modified (and streamlined) by natural selection for 200 million years. According to current dogma, the archedictyon contained six to eight longitudinal veins. These veins (and their branches) are named according to a system devised by John Comstock and George Needham—the Comstock-Needham system:[37]
- Costa (C) - the leading edge of the wing
- Subcosta (Sc) - second longitudinal vein (behind the costa), typically unbranched
- Radius (R) - third longitudinal vein, one to five branches reach the wing margin
- Media (M) - fourth longitudinal vein, one to four branches reach the wing margin
- Cubitus (Cu) - fifth longitudinal vein, one to three branches reach the wing margin
- Anal veins (A1, A2, A3) - unbranched veins behind the cubitus
The costa (C) is the leading marginal vein on most insects, although a small vein, the precosta, is sometimes found above the costa. In almost all extant insects,[1]:41–42 the precosta is fused with the costa; the costa rarely ever branches because it is at the leading edge, which is associated at its base with the humeral plate. The trachea of the costal vein is perhaps a branch of the subcostal trachea. Located after the costa is the third vein, the subcosta, which branches into two separate veins: the anterior and posterior. The base of the subcosta is associated with the distal end of the neck of the first axillary. The fourth vein is the radius, which is branched into five separate veins. The radius is generally the strongest vein of the wing. Toward the middle of the wing, it forks into a first undivided branch (R1) and a second branch, called the radial sector (Ra), which subdivides dichotomously into four distal branches (R2, R3, R4, R5). Basally, the radius is flexibly united with the anterior end of the second axillary (2Ax).[38]
The fifth vein of the wing is the media. In the archetype pattern (A), the media forks into two main branches, a media anterior (MA), which divides into two distal branches (MA1, MA2), and a median sector, or media posterior (MP), which has four terminal branches (M1, M2, M3, M4). In most modern insects, the media anterior has been lost, and the usual "media" is the four-branched media posterior with the common basal stem. In the Ephemerida, according to present interpretations of the wing venation, both branches of the media are retained, while in Odonata, the persisting media is the primitive anterior branch. The stem of the media is often united with the radius, but when it occurs as a distinct vein, its base is associated with the distal median plate (m') or is continuously sclerotized with the latter. The cubitus, the sixth vein of the wing, is primarily two-branched. The primary forking takes place near the base of the wing, forming the two principal branches (Cu1, Cu2). The anterior branch may break up into a number of secondary branches, but commonly it forks into two distal branches. The second branch of the cubitus (Cu2) in Hymenoptera, Trichoptera, and Lepidoptera, was mistaken by Comstock and Needham for the first anal. Proximally, the main stem of the cubitus is associated with the distal median plate (m') of the wing base.[38]
The postcubitus (Pcu) is the first anal of the Comstock and Needham system. The postcubitus, however, has the status of an independent wing vein and should be recognized as such. In nymphal wings, its trachea arises between the cubital trachea and the group of vannal tracheae. In the mature wings of more generalized insects, the postcubitus is always associated proximally with the cubitus, and is never intimately connected with the flexor sclerite (3Ax) of the wing base. In Neuroptera, Mecoptera, and Trichoptera, the postcubitus may be more closely associated with the vannal veins, but its base is always free from the latter. The postcubitus is usually unbranched; primitively, it is two-branched. The vannal veins (lV to nV) are the anal veins immediately associated with the third axillary, and which are directly affected by the movement of this sclerite that brings about the flexion of the wings. In number, the vannal veins vary from one to 12, according to the expansion of the vannal area of the wing. The vannal tracheae usually arise from a common tracheal stem in nymphal insects, and the veins are regarded as branches of a single anal vein. Distally, the vannal veins are either simple or branched. The jugal vein (J) of the jugal lobe of the wing is often occupied by a network of irregular veins, or it may be entirely membranous; sometimes it contains one or two distinct, small veins, the first jugal vein, or vena arcuata, and the second jugal vein, or vena cardinalis (2J).[38]
- C-Sc cross-veins - run between the costa and subcosta
- R cross-veins - run between adjacent branches of the radius
- R-M cross-veins - run between the radius and media
- M-Cu cross-veins - run between the media and cubitus
All the veins of the wing are subject to secondary forking and to union by cross-veins. In some orders of insects, the cross-veins are so numerous, the whole venational pattern becomes a close network of branching veins and cross-veins. Ordinarily, however, a definite number of cross-veins having specific locations occurs. The more constant cross-veins are the humeral cross-vein (h) between the costa and subcosta, the radial cross-vein (r) between R and the first fork of Rs, the sectorial cross-vein (s) between the two forks of R8, the median cross-vein (m-m) between M2 and M3, and the mediocubital cross-vein (m-cu) between the media and the cubitus.[38]
The veins of insect wings are characterized by a convex-concave placement, such as those seen in mayflies (i.e., concave is "down" and convex is "up"), which alternate regularly and by their branching; whenever a vein forks there is always an interpolated vein of the opposite position between the two branches. The concave vein will fork into two concave veins (with the interpolated vein being convex) and the regular alteration of the veins is preserved.[39] The veins of the wing appear to fall into an undulating pattern according to whether they have a tendency to fold up or down when the wing is relaxed. The basal shafts of the veins are convex, but each vein forks distally into an anterior convex branch and a posterior concave branch. Thus, the costa and subcosta are regarded as convex and concave branches of a primary first vein, Rs is the concave branch of the radius, posterior media the concave branch of the media, Cu1 and Cu2 are respectively convex and concave, while the primitive postcubitus and the first vannal have each an anterior convex branch and a posterior concave branch. The convex or concave nature of the veins has been used as evidence in determining the identities of the persisting distal branches of the veins of modern insects, but it has not been demonstrated to be consistent for all wings.[26][38]
Fields
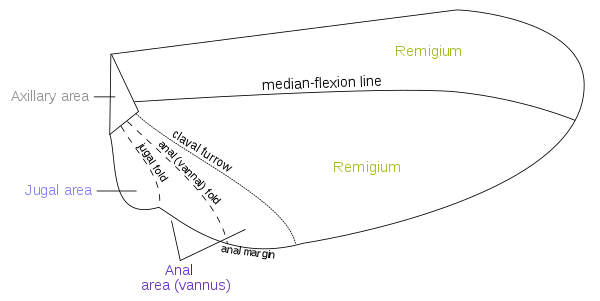
Wing areas are delimited and subdivided by fold lines, along which the wings can fold, and flexion lines, which flex during flight. Between the flexion and the fold lines, the fundamental distinction is often blurred, as fold lines may permit some flexibility or vice versa. Two constants, found in nearly all insect wings, are the claval (a flexion line) and jugal folds (or fold line), forming variable and unsatisfactory boundaries. Wing foldings can very complicated, with transverse folding occurring in the hindwings of Dermaptera and Coleoptera, and in some insects, the anal area can be folded like a fan.[1]:41–42 The four different fields found on insect wings are:
- Remigium
- Anal area (vannus)
- Jugal area
- Axillary area
- Alula
Most veins and cross-veins occur in the anterior area of the remigium, which is responsible for most of the flight, powered by the thoracic muscles. The posterior portion of the remigium is sometimes called the clavus; the two other posterior fields are the anal and jugal areas.[1]:41–42 When the vannal fold has the usual position anterior to the group of anal veins, the remigium contains the costal, subcostal, radial, medial, cubital, and postcubital veins. In the flexed wing, the remigium turns posteriorly on the flexible basal connection of the radius with the second axillary, and the base of the mediocubital field is folded medially on the axillary region along the plica basalis (bf) between the median plates (m, m') of the wing base.[38]
The vannus is bordered by the vannal fold, which typically occurs between the postcubitus and the first vannal vein. In Orthoptera, it usually has this position. In the forewing of Blattidae, however, the only fold in this part of the wing lies immediately before the postcubitus. In Plecoptera, the vannal fold is posterior to the postcubitus, but proximally it crosses the base of the first vannal vein. In the cicada, the vannal fold lies immediately behind the first vannal vein (lV). These small variations in the actual position of the vannal fold, however, do not affect the unity of action of the vannal veins, controlled by the flexor sclerite (3Ax), in the flexion of the wing. In the hindwings of most Orthoptera, a secondary vena dividens forms a rib in the vannal fold. The vannus is usually triangular in shape, and its veins typically spread out from the third axillary like the ribs of a fan. Some of the vannal veins may be branched, and secondary veins may alternate with the primary veins. The vannal region is usually best developed in the hindwing, in which it may be enlarged to form a sustaining surface, as in Plecoptera and Orthoptera. The great fan-like expansions of the hindwings of Acrididae are clearly the vannal regions, since their veins are all supported on the third axillary sclerites on the wing bases, though Martynov (1925) ascribes most of the fan areas in Acrididae to the jugal regions of the wings. The true jugum of the acridid wing is represented only by the small membrane (Ju) mesad of the last vannal vein. The jugum is more highly developed in some other Orthoptera, as in the Mantidae. In most of the higher insects with narrow wings, the vannus becomes reduced, and the vannal fold is lost, but even in such cases, the flexed wing may bend along a line between the postcubitus and the first vannal vein.[38]
The jugal region, or neala, is a region of the wing that is usually a small membranous area proximal to the base of the vannus strengthened by a few small, irregular vein-like thickenings; but when well developed, it is a distinct section of the wing and may contain one or two jugal veins. When the jugal area of the forewing is developed as a free lobe, it projects beneath the humeral angle of the hindwing and thus serves to yoke the two wings together. In the Jugatae group of Lepidoptera, it bears a long finger-like lobe. The jugal region was termed the neala ("new wing") because it is evidently a secondary and recently developed part of the wing.[38]
The auxiliary region containing the axillary sclerites has, in general, the form of a scalene triangle. The base of the triangle (a-b) is the hinge of the wing with the body; the apex (c) is the distal end of the third axillary sclerite; the longer side is anterior to the apex. The point d on the anterior side of the triangle marks the articulation of the radial vein with the second axillary sclerite. The line between d and c is the plica basalis (bf), or fold of the wing at the base of the mediocubital field.[38]
At the posterior angle of the wing base in some Diptera there is a pair of membranous lobes (squamae, or calypteres) known as the alula. The alula is well developed in the house fly. The outer squama (c) arises from the wing base behind the third axillary sclerite (3Ax) and evidently represents the jugal lobe of other insects (A, D); the larger inner squama (d) arises from the posterior scutellar margin of the tergum of the wing-bearing segment and forms a protective, hood-like canopy over the halter. In the flexed wing, the outer squama of the alula is turned upside down above the inner squama, the latter not being affected by the movement of the wing. In many Diptera, a deep incision of the anal area of the wing membrane behind the single vannal vein sets off a proximal alar lobe distal to the outer squama of the alula.[38]
Joints
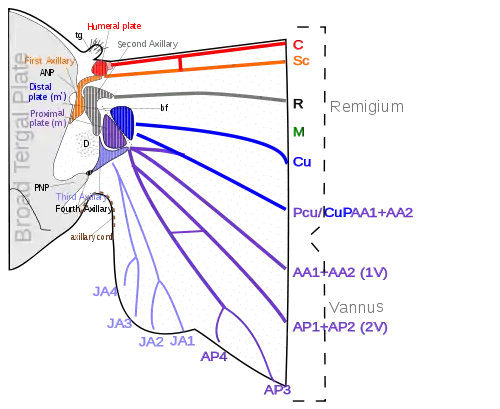
The various movements of the wings, especially in insects that flex their wings horizontally over their backs when at rest, demand a more complicated articular structure at the wing base than a mere hinge of the wing with the body. Each wing is attached to the body by a membranous basal area, but the articular membrane contains a number of small articular sclerites, collectively known as the pteralia. The pteralia include an anterior humeral plate at the base of the costal vein, a group of axillaries (Ax) associated with the subcostal, radial, and vannal veins, and two less definite median plates (m, m') at the base of the mediocubital area. The axillaries are specifically developed only in the wing-flexing insects, where they constitute the flexor mechanism of the wing operated by the flexor muscle arising on the pleuron. Characteristic of the wing base is also a small lobe on the anterior margin of the articular area proximal to the humeral plate, which, in the forewing of some insects, is developed into a large, flat, scale-like flap, the tegula, overlapping the base of the wing. Posteriorly, the articular membrane often forms an ample lobe between the wing and the body, and its margin is generally thickened and corrugated, giving the appearance of a ligament, the so-called axillary cord, continuous mesally with the posterior marginal scutellar fold of the tergal plate bearing the wing.[38]
The articular sclerites, or pteralia, of the wing base of the wing-flexing insects and their relations to the body and the wing veins, shown diagrammatically, are as follows:
- Humeral plates
- First Axillary
- Second Axillary
- Third Axillary
- Fourth Axillary
- Median plates (m, m')
The humeral plate is usually a small sclerite on the anterior margin of the wing base, movable and articulated with the base of the costal vein. Odonata have their humeral plates greatly enlargened,[38] with two muscles arising from the episternum inserted into the humeral plates and two from the edge of the epimeron inserted into the axillary plate.[26]
The first axillary sclerite (lAx) is the anterior hinge plate of the wing base. Its anterior part is supported on the anterior notal wing process of the tergum (ANP); its posterior part articulates with the tergal margin. The anterior end of the sclerite is generally produced as a slender arm, the apex of which (e) is always associated with the base of the subcostal vein (Sc), though it is not united with the latter. The body of the sclerite articulates laterally with the second axillary. The second axillary sclerite (2Ax) is more variable in form than the first axillary, but its mechanical relations are no less definite. It is obliquely hinged to the outer margin of the body of the first axillary, and the radial vein (R) is always flexibly attached to its anterior end (d). The second axillary presents both a dorsal and a ventral sclerotization in the wing base; its ventral surface rests upon the fulcral wing process of the pleuron. The second axillary, therefore, is the pivotal sclerite of the wing base, and it specifically manipulates the radial vein.[38]
The third axillary sclerite (3Ax) lies in the posterior part of the articular region of the wing. Its form is highly variable and often irregular, but the third axillary is the sclerite on which is inserted the flexor muscle of the wing (D). Mesally, it articulates anteriorly (f) with the posterior end of the second axillary, and posteriorly (b) with the posterior wing process of the tergum (PNP), or with a small fourth axillary when the latter is present. Distally, the third axillary is prolonged in a process always associated with the bases of the group of veins in the anal region of the wing, here termed the vannal veins (V). The third axillary, therefore, is usually the posterior hinge plate of the wing base and is the active sclerite of the flexor mechanism, which directly manipulates the vannal veins. The contraction of the flexor muscle (D) revolves the third axillary on its mesal articulations (b, f), and thereby lifts its distal arm; this movement produces the flexion of the wing. The fourth axillary sclerite is not a constant element of the wing base. When present, it is usually a small plate intervening between the third axillary and the posterior notal wing process, and is probably a detached piece of the latter.[38]
The median plates (m, m') are also sclerites that are not so definitely differentiated as specific plates as are the three principal axillaries, but they are important elements of the flexor apparatus. They lie in the median area of the wing base distal to the second and third axillaries, and are separated from each other by an oblique line (bf), which forms a prominent convex fold during flexion of the wing. The proximal plate (m) is usually attached to the distal arm of the third axillary and perhaps should be regarded as a part of the latter. The distal plate (m') is less constantly present as a distinct sclerite, and may be represented by a general sclerotization of the base of the mediocubital field of the wing. When the veins of this region are distinct at their bases, they are associated with the outer median plate.[38]
Coupling, folding, and other features
In many insect species, the forewing and hindwing are coupled together, which improves the aerodynamic efficiency of flight. The most common coupling mechanism (e.g., Hymenoptera and Trichoptera) is a row of small hooks on the forward margin of the hindwing, or "hamuli", which lock onto the forewing, keeping them held together (hamulate coupling). In some other insect species (e.g., Mecoptera, Lepidoptera, and some Trichoptera) the jugal lobe of the forewing covers a portion of the hindwing (jugal coupling), or the margins of the forewing and hindwing overlap broadly (amplexiform coupling), or the hindwing bristles, or frenulum, hook under the retaining structure or retinalucum on the forewing.[1]:43
When at rest, the wings are held over the back in most insects, which may involve longitudinal folding of the wing membrane and sometimes also transverse folding. Folding may sometimes occur along the flexion lines. Though fold lines may be transverse, as in the hindwings of beetles and earwigs, they are normally radial to the base of the wing, allowing adjacent sections of a wing to be folded over or under each other. The commonest fold line is the jugal fold, situated just behind the third anal vein,[27] although, most Neoptera have a jugal fold just behind vein 3A on the forewings. It is sometimes also present on the hindwings. Where the anal area of the hindwing is large, as in Orthoptera and Blattodea, the whole of this part may be folded under the anterior part of the wing along a vannal fold a little posterior to the claval furrow. In addition, in Orthoptera and Blattodea, the anal area is folded like a fan along the veins, the anal veins being convex, at the crests of the folds, and the accessory veins concave. Whereas the claval furrow and jugal fold are probably homologous in different species, the vannal fold varies in position in different taxa. Folding is produced by a muscle arising on the pleuron and inserted into the third axillary sclerite in such a waythat, when it contracts, the sclerite pivots about its points of articulation with the posterior notal process and the second axillary sclerite.[26]
As a result, the distal arm of the third axillary sclerite rotates upwards and inwards, so that finally its position is completely reversed. The anal veins are articulated with this sclerite in such a way that when it moves they are carried with it and become flexed over the back of the insect. Activity of the same muscle in flight affects the power output of the wing and so it is also important in flight control. In orthopteroid insects, the elasticity of the cuticle causes the vannal area of the wing to fold along the veins. Consequently, energy is expended in unfolding this region when the wings are moved to the flight position. In general, wing extension probably results from the contraction of muscles attached to the basilar sclerite or, in some insects, to the subalar sclerite.[26]
Legs
The typical and usual segments of the insect leg are divided into the coxa, one trochanter, the femur, the tibia, the tarsus, and the pretarsus. The coxa in its more symmetrical form, has the shape of a short cylinder or truncate cone, though commonly it is ovate and may be almost spherical. The proximal end of the coxa is girdled by a submarginal basicostal suture that forms internally a ridge, or basicosta, and sets off a marginal flange, the coxomarginale, or basicoxite. The basicosta strengthens the base of the coxa and is commonly enlarged on the outer wall to give insertion to muscles; on the mesal half of the coxa, however, it is usually weak and often confluent with the coxal margin. The trochanteral muscles that take their origin in the coxa are always attached distal to the basicosta. The coxa is attached to the body by an articular membrane, the coxal corium, which surrounds its base. These two articulations are perhaps the primary dorsal and ventral articular points of the subcoxo-coxal hinge. In addition, the insect coxa has often an anterior articulation with the anterior, ventral end of the trochantin, but the trochantinal articulation does not coexist with a sternal articulation. The pleural articular surface of the coxa is borne on a mesal inflection of the coxal wall. If the coxa is movable on the pleural articulation alone, the coxal articular surface is usually inflected to a sufficient depth to give a leverage to the abductor muscles inserted on the outer rim of the coxal base. Distally the coxa bears an anterior and a posterior articulation with the trochanter. The outer wall of the coxa is often marked by a suture extending from the base to the anterior trochanteral articulation. In some insects the coxal suture falls in line with the pleural suture, and in such cases the coxa appears to be divided into two parts corresponding to the episternum and epimeron of the pleuron. The coxal suture is absent in many insects.[38]:163–164
The inflection of the coxal wall bearing the pleural articular surface divides the lateral wall of the basicoxite into a prearticular part and a postarticular part, and the two areas often appear as two marginal lobes on the base of the coxa. The posterior lobe is usually the larger and is termed the meron. The meron may be greatly enlarged by an extension distally in the posterior wall of the coxa; in the Neuroptera, Mecoptera, Trichoptera, and Lepidoptera, the meron is so large that the coxa appears to be divided into an anterior piece, the so-called "coxa genuina," and the meron, but the meron never includes the region of the posterior trochanteral articulation, and the groove delimiting it is always a part of the basicostal suture. A coxa with an enlarged meron has an appearance similar to one divided by a coxal suture falling in line with the pleural suture, but the two conditions are fundamentally quite different and should not be confused. The meron reaches the extreme of its departure from the usual condition in the Diptera. In some of the more generalized flies, as in the Tipulidae, the meron of the middle leg appears as a large lobe of the coxa projecting upward and posteriorly from the coxal base; in higher members of the order it becomes completely separated from the coxa and forms a plate of the lateral wall of the mesothorax.[38]:164
The trochanter is the basal segment of the telopodite; it is always a small segment in the insect leg, freely movable by a horizontal hinge on the coxa, but more or less fixed to the base of the femur. When movable on the femur the trochantero femoral hinge is usually vertical or oblique in a vertical plane, giving a slight movement of production and reduction at the joint, though only a reductor muscle is present. In the Odonata, both nymphs and adults, there are two trochanteral segments, but they are not movable on each other; the second contains the reductor muscle of the femur. The usual single trochanteral segment of insects, therefore, probably represents the two trochanters of other arthropods fused into one apparent segment, since it is not likely that the primary coxotrochanteral hinge has been lost from the leg. In some of the Hymenoptera a basal subdivision of the femur simulates a second trochanter, but the insertion of the reductor muscle on its base attests that it belongs to the femoral segment, since as shown in the odonate leg, the reductor has its origin in the true second trochanter.[38]:165
The femur is the third segment of the insect leg, is usually the longest and strongest part of the limb, but it varies in size from the huge hind femur of leaping Orthoptera to a very small segment such as is present in many larval forms. The volume of the femur is generally correlated with the size of the tibial muscles contained within it, but it is sometimes enlarged and modified in shape for other purposes than that of accommodating the tibial muscles. The tibia is characteristically a slender segment in adult insects, only a little shorter than the femur or the combined femur and trochanter. Its proximal end forms a more or less distinct head bent toward the femur, a device allowing the tibia to be flexed close against the under surface of the femur.[38]:165
The terms profemur, mesofemur and metafemur refer to the femora of the front, middle and hind legs of an insect, respectively.[40] Similarly protibia, mesotibia and metatibia refer to the tibiae of the front, middle and hind legs.[41]
The tarsus of insects corresponds to the penultimate segment of a generalized arthropod limb, which is the segment called the propodite in Crustacea. In adult insects it is commonly subdivided into from two to five subsegments, or tarsomeres, but in the Protura, some Collembola, and most holometabolous insect larvae it preserves the primitive form of a simple segment. The subsegments of the adult insect tarsus are usually freely movable on one another by inflected connecting membranes, but the tarsus never has intrinsic muscles. The tarsus of adult pterygote insects having fewer than five subsegments is probably specialized by the loss of one or more subsegments or by a fusion of adjoining subsegments. In the tarsi of Acrididae the long basal piece is evidently composed of three united tarsomeres, leaving the fourth and the fifth. The basal tarsomere is sometimes conspicuously enlarged and is distinguished as the basitarsus. On the under surfaces of the tarsal subsegments in certain Orthoptera there are small pads, the tarsal pulvilli, or euplantulae. The tarsus is occasionally fused with the tibia in larval insects, forming a tibiotarsal segment; in some cases it appears to be eliminated or reduced to a rudiment between the tibia and the pretarsus.[38]:165–166
For the most part the femur and tibia are the longest leg segments but variations in the lengths and robustness of each segment relate to their functions. For example, gressorial and cursorial, or walking and running type insects respectively, usually have well-developed femora and tibiae on all legs, whereas jumping (saltatorial) insects such as grasshoppers have disproportionately developed metafemora and metatibiae. In aquatic beetles (Coleoptera) and bugs (Hemiptera), the tibiae and/or tarsi of one or more pairs of legs usually are modified for swimming (natatorial) with fringes of long, slender hairs. Many ground-dwelling insects, such as mole crickets (Orthoptera: Gryllotalpidae), nymphal cicadas (Hemiptera: Cicadidae), and scarab beetles (Scarabaeidae), have the tibiae of the forelegs (protibiae) enlarged and modified for digging (fossorial), whereas the forelegs of some predatory insects, such as mantispid lacewings (Neuroptera) and mantids (Mantodea), are specialized for seizing prey, or raptorial. The tibia and basal tarsomere of each hindleg of honey bees are modified for the collection and carriage of pollen.[26]:45
Abdomen
The ground plan of the abdomen of an adult insect typically consists of 11–12 segments and is less strongly sclerotized than the head or thorax. Each segment of the abdomen is represented by a sclerotized tergum, sternum, and perhaps a pleurite. Terga are separated from each other and from the adjacent sterna or pleura by a membrane. Spiracles are located in the pleural area. Variation of this ground plan includes the fusion of terga or terga and sterna to form continuous dorsal or ventral shields or a conical tube. Some insects bear a sclerite in the pleural area called a laterotergite. Ventral sclerites are sometimes called laterosternites. During the embryonic stage of many insects and the postembryonic stage of primitive insects, 11 abdominal segments are present. In modern insects there is a tendency toward reduction in the number of the abdominal segments, but the primitive number of 11 is maintained during embryogenesis. Variation in abdominal segment number is considerable. If the Apterygota are considered to be indicative of the ground plan for pterygotes, confusion reigns: adult Protura have 12 segments, Collembola have 6. The orthopteran family Acrididae has 11 segments, and a fossil specimen of Zoraptera has a 10-segmented abdomen.[8]
Generally, the first seven abdominal segments of adults (the pregenital segments) are similar in structure and lack appendages. However, apterygotes (bristletails and silverfish) and many immature aquatic insects have abdominal appendages. Apterygotes possess a pair of styles; rudimentary appendages that are serially homologous with the distal part of the thoracic legs. And, mesally, one or two pairs of protrusible (or exsertile) vesicles on at least some abdominal segments. These vesicles are derived from the coxal and trochanteral endites (inner annulated lobes) of the ancestral abdominal appendages. Aquatic larvae and nymphs may have gills laterally on some to most abdominal segments.[1]:49 Of the rest of the abdominal segments consist of the reproductive and anal parts.
The anal-genital part of the abdomen, known as the terminalia, consists generally of segments 8 or 9 to the abdominal apex. Segments 8 and 9 bear the genitalia; segment 10 is visible as a complete segment in many "lower" insects but always lacks appendages; and the small segment 11 is represented by a dorsal epiproct and pair of ventral paraprocts derived from the sternum. A pair of appendages, the cerci, articulates laterally on segment 11; typically these are annulated and filamentous but have been modified (e.g. the forceps of earwigs) or reduced in different insect orders. An annulated caudal filament, the median appendix dorsalis, arises from the tip of the epiproct in apterygotes, most mayflies (Ephemeroptera), and a few fossil insects. A similar structure in nymphal stoneflies (Plecoptera) is of uncertain homology. These terminal abdominal segments have excretory and sensory functions in all insects, but in adults there is an additional reproductive function.[1]:49
External genitalia

The organs concerned specifically with mating and the deposition of eggs are known collectively as the external genitalia, although they may be largely internal. The components of the external genitalia of insects are very diverse in form and often have considerable taxonomic value, particularly among species that appear structurally similar in other respects. The male external genitalia have been used widely to aid in distinguishing species, whereas the female external genitalia may be simpler and less varied.
The terminalia of adult female insects include internal structures for receiving the male copulatory organ and his spermatozoa and external structures used for oviposition (egg-laying; section 5.8). Most female insects have an egg-laying tube, or ovipositor; it is absent in termites, parasitic lice, many Plecoptera, and most Ephemeroptera. Ovipositors take two forms:
- true, or appendicular, formed from appendages of abdominal segments 8 and 9;
- substitutional, composed of extensible posterior abdominal segments.
Other Appendages
Internal
Nervous system
The nervous system of an insect can be divided into a brain and a ventral nerve cord. The head capsule is made up of six fused segments, each with a pair of ganglia, or a cluster of nerve cells outside of the brain. The first three pairs of ganglia are fused into the brain, while the three following pairs are fused into a structure of three pairs of ganglia under the insect's esophagus, called the subesophageal ganglion.[1]:57
The thoracic segments have one ganglion on each side, which are connected into a pair, one pair per segment. This arrangement is also seen in the abdomen but only in the first eight segments. Many species of insects have reduced numbers of ganglia due to fusion or reduction.[42] Some cockroaches have just six ganglia in the abdomen, whereas the wasp Vespa crabro has only two in the thorax and three in the abdomen. Some insects, like the house fly Musca domestica, have all the body ganglia fused into a single large thoracic ganglion.
At least a few insects have nociceptors, cells that detect and transmit sensations of pain.[43] This was discovered in 2003 by studying the variation in reactions of larvae of the common fruitfly Drosophila to the touch of a heated probe and an unheated one. The larvae reacted to the touch of the heated probe with a stereotypical rolling behavior that was not exhibited when the larvae were touched by the unheated probe.[44] Although nociception has been demonstrated in insects, there is not a consensus that insects feel pain consciously.[45]
Digestive system
An insect uses its digestive system for all steps in food processing: digestion, absorption, and feces delivery and elimination.[46][47] Most of this food is ingested in the form of macromolecules and other complex substances like proteins, polysaccharides, fats, and nucleic acids. These macromolecules must be broken down by catabolic reactions into smaller molecules like amino acids and simple sugars before being used by cells of the body for energy, growth, or reproduction. This break-down process is known as digestion. The main structure of an insect's digestive system is a long enclosed tube called the alimentary canal (or gut), which runs lengthwise through the body. The alimentary canal directs food in one direction: from the mouth to the anus. The gut is where almost all of insects' digestion takes place. It can be divided into three sections - the foregut, midgut and hindgut - each of which performs a different process of digestion. [48] In addition to the alimentary canal, insects also have paired salivary glands and salivary reservoirs. These structures usually reside in the thorax, adjacent to the foregut.[1]:70–77
Foregut
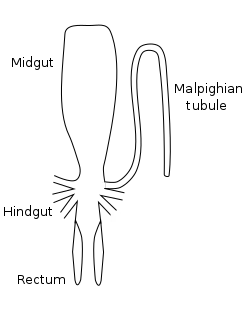
The first section of the alimentary canal is the foregut (element 27 in numbered diagram), or stomodaeum. The foregut is lined with a cuticular lining made of chitin and proteins as protection from tough food. The foregut includes the buccal cavity (mouth), pharynx, esophagus, and Crop and proventriculus (any part may be highly modified), which both store food and signify when to continue passing onward to the midgut.[1]:70 Here, digestion starts as partially chewed food is broken down by saliva from the salivary glands. As the salivary glands produce fluid and carbohydrate-digesting enzymes (mostly amylases), strong muscles in the pharynx pump fluid into the buccal cavity, lubricating the food like the salivarium does, and helping blood feeders, and xylem and phloem feeders.
From there, the pharynx passes food to the esophagus, which could be just a simple tube passing it on to the crop and proventriculus, and then on ward to the midgut, as in most insects. Alternately, the foregut may expand into a very enlarged crop and proventriculus, or the crop could just be a diverticulum, or fluid filled structure, as in some Diptera species.[49]:30–31
The salivary glands (element 30 in numbered diagram) in an insect's mouth produce saliva. The salivary ducts lead from the glands to the reservoirs and then forward through the head to an opening called the salivarium, located behind the hypopharynx. By moving its mouthparts (element 32 in numbered diagram) the insect can mix its food with saliva. The mixture of saliva and food then travels through the salivary tubes into the mouth, where it begins to break down.[46][50] Some insects, like flies, have extra-oral digestion. Insects using extra-oral digestion expel digestive enzymes onto their food to break it down. This strategy allows insects to extract a significant proportion of the available nutrients from the food source.[49]:31
Midgut
Once food leaves the crop, it passes to the midgut (element 13 in numbered diagram), also known as the mesenteron, where the majority of digestion takes place. Microscopic projections from the midgut wall, called microvilli, increase the surface area of the wall and allow more nutrients to be absorbed; they tend to be close to the origin of the midgut. In some insects, the role of the microvilli and where they are located may vary. For example, specialized microvilli producing digestive enzymes may more likely be near the end of the midgut, and absorption near the origin or beginning of the midgut.[49]:32
Hindgut
In the hindgut (element 16 in numbered diagram), or proctodaeum, undigested food particles are joined by uric acid to form fecal pellets. The rectum absorbs 90% of the water in these fecal pellets, and the dry pellet is then eliminated through the anus (element 17), completing the process of digestion. The uric acid is formed using hemolymph waste products diffused from the Malpighian tubules (element 20). It is then emptied directly into the alimentary canal, at the junction between the midgut and hindgut. The number of Malpighian tubules possessed by a given insect varies between species, ranging from only two tubules in some insects to over 100 tubules in others.[1]:71–72, 78–80
Respiratory systems
Insect respiration is accomplished without lungs. Instead, the insect respiratory system uses a system of internal tubes and sacs through which gases either diffuse or are actively pumped, delivering oxygen directly to tissues that need it via their trachea (element 8 in numbered diagram). Since oxygen is delivered directly, the circulatory system is not used to carry oxygen, and is therefore greatly reduced. The insect circulatory system has no veins or arteries, and instead consists of little more than a single, perforated dorsal tube that pulses peristaltically. Toward the thorax, the dorsal tube (element 14) divides into chambers and acts like the insect's heart. The opposite end of the dorsal tube is like the aorta of the insect circulating the hemolymph, arthropods' fluid analog of blood, inside the body cavity.[1]:61–65[51] Air is taken in through openings on the sides of the abdomen called spiracles.
There are many different patterns of gas exchange demonstrated by different groups of insects. Gas exchange patterns in insects can range from continuous and diffusive ventilation, to discontinuous gas exchange.[1]:65–68 During continuous gas exchange, oxygen is taken in and carbon dioxide is released in a continuous cycle. In discontinuous gas exchange, however, the insect takes in oxygen while it is active and small amounts of carbon dioxide are released when the insect is at rest.[52] Diffusive ventilation is simply a form of continuous gas exchange that occurs by diffusion rather than physically taking in the oxygen. Some species of insect that are submerged also have adaptations to aid in respiration. As larvae, many insects have gills that can extract oxygen dissolved in water, while others need to rise to the water surface to replenish air supplies, which may be held or trapped in special structures.[53][54]
Circulatory system
Insect blood or haemolymph's main function is that of transport and it bathes the insect's body organs. Making up usually less than 25% of an insect's body weight, it transports hormones, nutrients and wastes and has a role in, osmoregulation, temperature control, immunity, storage (water, carbohydrates and fats) and skeletal function. It also plays an essential part in the moulting process.[55][56] An additional role of the haemolymph in some orders, can be that of predatory defence. It can contain unpalatable and malodourous chemicals that will act as a deterrent to predators.[1] Haemolymph contains molecules, ions and cells;[1] regulating chemical exchanges between tissues, haemolymph is encased in the insect body cavity or haemocoel.[1][57] It is transported around the body by combined heart (posterior) and aorta (anterior) pulsations, which are located dorsally just under the surface of the body.[1][55][56] It differs from vertebrate blood in that it doesn't contain any red blood cells and therefore is without high oxygen carrying capacity, and is more similar to lymph found in vertebrates.[1][57]
Body fluids enter through one-way valved ostia, which are openings situated along the length of the combined aorta and heart organ. Pumping of the haemolymph occurs by waves of peristaltic contraction, originating at the body's posterior end, pumping forwards into the dorsal vessel, out via the aorta and then into the head where it flows out into the haemocoel.[1][57] The haemolymph is circulated to the appendages unidirectionally with the aid of muscular pumps or accessory pulsatile organs usually found at the base of the antennae or wings and sometimes in the legs,[1] with pumping rates accelerating with periods of increased activity.[56] Movement of haemolymph is particularly important for thermoregulation in orders such as Odonata, Lepidoptera, Hymenoptera and Diptera.[1]
Endocrine system
These glands are part of the endocrine system:
1. Neurosecretory cells
2. Corpora cardiaca
Reproductive system
Female
Female insects are able make eggs, receive and store sperm, manipulate sperm from different males, and lay eggs. Their reproductive systems are made up of a pair of ovaries, accessory glands, one or more spermathecae, and ducts connecting these parts. The ovaries make eggs and accessory glands produce the substances to help package and lay the eggs. Spermathecae store sperm for varying periods of time and, along with portions of the oviducts, can control sperm use. The ducts and spermathecae are lined with a cuticle.[8]:880
The ovaries are made up of a number of egg tubes, called ovarioles, which vary in size and number by species. The number of eggs that the insect is able to make vary by the number of ovarioles with the rate that eggs can be developed being also influenced by ovariole design. In meroistic ovaries, the eggs-to-be divide repeatedly and most of the daughter cells become helper cells for a single oocyte in the cluster. In panoistic ovaries, each egg-to-be produced by stem germ cells develops into an oocyte; there are no helper cells from the germ line. Production of eggs by panoistic ovaries tends to be slower than that by meroistic ovaries.[8]:880
Accessory glands or glandular parts of the oviducts produce a variety of substances for sperm maintenance, transport, and fertilization, as well as for protection of eggs. They can produce glue and protective substances for coating eggs or tough coverings for a batch of eggs called oothecae. Spermathecae are tubes or sacs in which sperm can be stored between the time of mating and the time an egg is fertilized. Paternity testing of insects has revealed that some, and probably many, female insects use the spermatheca and various ducts to control or bias sperm used in favor of some males over others.[8]:880
Male
The main component of the male reproductive system is the testis, suspended in the body cavity by tracheae and the fat body. The more primitive apterygote insects have a single testis, and in some lepidopterans the two maturing testes are secondarily fused into one structure during the later stages of larval development, although the ducts leading from them remain separate. However, most male insects have a pair of testes, inside of which are sperm tubes or follicles that are enclosed within a membranous sac. The follicles connect to the vas deferens by the vas efferens, and the two tubular vasa deferentia connect to a median ejaculatory duct that leads to the outside. A portion of the vas deferens is often enlarged to form the seminal vesicle, which stores the sperm before they are discharged into the female. The seminal vesicles have glandular linings that secrete nutrients for nourishment and maintenance of the sperm. The ejaculatory duct is derived from an invagination of the epidermal cells during development and, as a result, has a cuticular lining. The terminal portion of the ejaculatory duct may be sclerotized to form the intromittent organ, the aedeagus. The remainder of the male reproductive system is derived from embryonic mesoderm, except for the germ cells, or spermatogonia, which descend from the primordial pole cells very early during embryogenesis.[8]:885 The aedeagus can be quite pronounced or de minimis. The base of the aedeagus may be the partially sclerotized phallotheca, also called the phallosoma or theca. In some species the phallotheca contains a space, called the endosoma (internal holding pouch), into which the tip end of the aedeagus may be withdrawn (retracted). The vas deferens is sometimes drawn into (folded into) the phallotheca together with a seminal vesicle.[60][61]
Internal morphology of different taxa
Blattodea
Cockroaches are most common in tropical and subtropical climates. Some species are in close association with human dwellings and widely found around garbage or in the kitchen. Cockroaches are generally omnivorous with the exception of the wood-eating species such as Cryptocercus; these roaches are incapable of digesting cellulose themselves, but have symbiotic relationships with various protozoans and bacteria that digest the cellulose, allowing them to extract the nutrients. The similarity of these symbionts in the genus Cryptocercus to those in termites are such that it has been suggested that they are more closely related to termites than to other cockroaches,[62] and current research strongly supports this hypothesis of relationships.[63] All species studied so far carry the obligate mutualistic endosymbiont bacterium Blattabacterium, with the exception of Nocticola australiensis, an Australian cave dwelling species without eyes, pigment or wings, and which recent genetic studies indicates are very primitive cockroaches.[64][65]
Cockroaches, like all insects, breathe through a system of tubes called tracheae. The tracheae of insects are attached to the spiracles, excluding the head. Thus cockroaches, like all insects, are not dependent on the mouth and windpipe to breathe. The valves open when the CO2 level in the insect rises to a high level; then the CO2 diffuses out of the tracheae to the outside and fresh O2 diffuses in. Unlike in vertebrates that depend on blood for transporting O2 and CO2, the tracheal system brings the air directly to cells, the tracheal tubes branching continually like a tree until their finest divisions, tracheoles, are associated with each cell, allowing gaseous oxygen to dissolve in the cytoplasm lying across the fine cuticle lining of the tracheole. CO2 diffuses out of the cell into the tracheole. While cockroaches do not have lungs and thus do not actively breathe in the vertebrate lung manner, in some very large species the body musculature may contract rhythmically to forcibly move air out and in the spiracles; this may be considered a form of breathing.[66]
Coleoptera
The digestive system of beetles is primarily based on plants, which they for the most part feed upon, with mostly the anterior midgut performing digestion. However, in predatory species (e.g., Carabidae) most digestion occurs in the crop by means of midgut enzymes. In Elateridae species, the predatory larvae defecate enzymes on their prey, with digestion being extraorally.[8] The alimentary canal basically comprises a short narrow pharynx, a widened expansion, the crop and a poorly developed gizzard. After there is a midgut, that varies in dimensions between species, with a large amount of cecum, with a hingut, with varying lengths. There are typically four to six Malpighian tubules.[67]
The nervous system in beetles contains all the types found in insects, varying between different species. With three thoracic and seven or eight abdominal ganglia can be distinguished to that in which all the thoracic and abdominal ganglia are fused to form a composite structure. Oxygen is obtained via a tracheal system. Air enters a series of tubes along the body through openings called spiracles, and is then taken into increasingly finer fibers.[8] Pumping movements of the body force the air through the system. Some species of diving beetles (Dytiscidae) carry a bubble of air with them whenever they dive beneath the water surface. This bubble may be held under the elytra or it may be trapped against the body using specialized hairs. The bubble usually covers one or more spiracles so the insect can breathe air from the bubble while submerged. An air bubble provides an insect with only a short-term supply of oxygen, but thanks to its unique physical properties, oxygen will diffuse into the bubble and displacing the nitrogen, called passive diffusion, however the volume of the bubble eventually diminishes and the beetle will have to return to the surface.[68]
Like other insect species, beetles have hemolymph instead of blood. The open circulatory system of the beetle is driven by a tube-like heart attached to the top inside of the thorax.
Different glands specialize for different pheromones produced for finding mates. Pheromones from species of Rutelinea are produced from epithelial cells lining the inner surface of the apical abdominal segments or amino acid based pheromones of Melolonthinae from eversible glands on the abdominal apex. Other species produce different types of pheromones. Dermestids produce esters, and species of Elateridae produce fatty-acid-derived aldehydes and acetates.[8] For means of finding a mate also, fireflies (Lampyridae) utilized modified fat body cells with transparent surfaces backed with reflective uric acid crystals to biosynthetically produce light, or bioluminescence. The light produce is highly efficient, as it is produced by oxidation of luciferin by the enzymes luciferase in the presence of ATP (adenosine triphospate) and oxygen, producing oxyluciferin, carbon dioxide, and light.[8]
A notable number of species have developed special glands that produce chemicals for deterring predators (see Defense and predation). The Ground beetle's (of Carabidae) defensive glands, located at the posterior, produce a variety of hydrocarbons, aldehydes, phenols, quinones, esters, and acids released from an opening at the end of the abdomen. While African carabid beetles (e.g., Anthia some of which used to comprise the genus Thermophilum) employ the same chemicals as ants: formic acid.[31] While Bombardier beetles have well developed, like other carabid beetles, pygidial glands that empty from the lateral edges of the intersegment membranes between the seventh and eighth abdominal segments. The gland is made of two containing chambers. The first holds hydroquinones and hydrogen peroxide, with the second holding just hydrogen peroxide plus catalases. These chemicals mix and result in an explosive ejection, forming temperatures of around 100 C, with the breakdown of hydroquinone to H2 + O2 + quinone, with the O2 propelling the excretion.[8]
Tympanal organs are hearing organs. Such an organ is generally a membrane (tympanum) stretched across a frame backed by an air sac and associated sensory neurons. In the order Coleoptera, tympanal organs have been described in at least two families.[30] Several species of the genus Cicindela in the family Cicindelidae have ears on the dorsal surface of the first abdominal segment beneath the wing; two tribes in the family Dynastinae (Scarabaeidae) have ears just beneath the pronotal shield or neck membrane. The ears of both families are to ultrasonic frequencies, with strong evidence that they function to detect the presence of bats via their ultrasonic echolocation. Even though beetles constitute a large order and live in a variety of niches, examples of hearing is surprisingly lacking in species, though it is likely that most are just undiscovered.[8]
Dermaptera
The neuroendocrine system is typical of insects. There is a brain, a subesophageal ganglion, three thoracic ganglia, and six abdominal ganglia. Strong neuron connections connect the neurohemal corpora cardiaca to the brain and frontal ganglion, where the closely related median corpus allatum produces juvenile hormone III in close proximity to the neurohemal dorsal aorta. The digestive system of earwigs is like all other insects, consisting of a fore-, mid-, and hindgut, but earwigs lack gastric caecae which are specialized for digestion in many species of insect. Long, slender (extratory) malpighian tubules can be found between the junction of the mid- and hind gut.[8]
The reproductive system of females consist of paired ovaries, lateral oviducts, spermatheca, and a genital chamber. The lateral ducts are where the eggs leave the body, while the spermatheca is where sperm is stored. Unlike other insects, the gonopore, or genital opening is behind the seventh abdominal segment. The ovaries are primitive in that they are polytrophic (the nurse cells and oocytes alternate along the length of the ovariole). In some species these long ovarioles branch off the lateral duct, while in others, short ovarioles appear around the duct.[8]
Diptera
The genitalia of female flies are rotated to a varying degree from the position found in other insects. In some flies this is a temporary rotation during mating, but in others it is a permanent torsion of the organs that occurs during the pupal stage. This torsion may lead to the anus being located below the genitals, or, in the case of 360° torsion, to the sperm duct being wrapped around the gut, despite the external organs being in their usual position. When flies mate, the male initially flies on top of the female, facing in the same direction, but then turns round to face in the opposite direction. This forces the male to lie on its back in order for its genitalia to remain engaged with those of the female, or the torsion of the male genitals allows the male to mate while remaining upright. This leads to flies having more reproduction abilities than most insects and at a much quicker rate. Flies come in great populations due to their ability to mate effectively and in a short period of time especially during the mating season.[69]
The female lays her eggs as close to the food source as possible, and development is very rapid, allowing the larva to consume as much food as possible in a short period of time before transforming into the adult. The eggs hatch immediately after being laid, or the flies are ovoviviparous, with the larva hatching inside the mother.[69] Larval flies, or maggots, have no true legs, and little demarcation between the thorax and abdomen; in the more derived species, the head is not clearly distinguishable from the rest of the body. Maggots are limbless, or else have small prolegs. The eyes and antennae are reduced or absent, and the abdomen also lacks appendages such as cerci. This lack of features is an adaptation to a food-rich environment, such as within rotting organic matter, or as an endoparasite.[69] The pupae take various forms, and in some cases develop inside a silk cocoon. After emerging from the pupa, the adult fly rarely lives more than a few days, and serves mainly to reproduce and to disperse in search of new food sources.
Lepidoptera
In reproductive system of butterflies and moths, the male genitalia are complex and unclear. In females there are three types of genitalia based on the relating taxa: monotrysian, exoporian, and dytresian. In the monotrysian type there is an opening on the fused segments of the sterna 9 and 10, which act as insemination and oviposition. In the exoporian type (in Hepialoidea and Mnesarchaeoidea) there are two separate places for insemination and oviposition, both occurring on the same sterna as the monotrysian type, 9/10. In most species the genitalia are flanked by two soft lobes, although they may be specialized and sclerotized in some species for ovipositing in area such as crevices and inside plant tissue.[67] Hormones and the glands that produce them run the development of butterflies and moths as they go through their life cycle, called the endocrine system. The first insect hormone PTTH (Prothoracicotropic hormone) operates the species life cycle and diapause (see the relates section).[70] This hormone is produced by corpora allata and corpora cardiaca, where it is also stored. Some glands are specialized to perform certain task such as producing silk or producing saliva in the palpi.[1]:65, 75 While the corpora cardiaca produce PTTH, the corpora allata also produces juvanile hormones, and the prothorocic glands produce moulting hormones.
In the digestive system, the anterior region of the foregut has been modified to form a pharyngeal sucking pump as they need it for the food they eat, which are for the most part liquids. An esophagus follows and leads to the posterior of the pharynx and in some species forms a form of crop. The midgut is short and straight, with the hindgut being longer and coiled.[67] Ancestors of lepidopteran species, stemming from Hymenoptera, had midgut ceca, although this is lost in current butterflies and moths. Instead, all the digestive enzymes other than initial digestion, are immobilized at the surface of the midgut cells. In larvae, long-necked and stalked goblet cells are found in the anterior and posterior midgut regions, respectively. In insects, the goblet cells excrete positive potassium ions, which are absorbed from leaves ingested by the larvae. Most butterflies and moths display the usual digestive cycle, however species that have a different diet require adaptations to meet these new demands.[8]:279
In the circulatory system, hemolymph, or insect blood, is used to circulate heat in a form of thermoregulation, where muscles contraction produces heat, which is transferred to the rest of the body when conditions are unfavorable.[71] In lepidopteran species, hemolymph is circulated through the veins in the wings by some form of pulsating organ, either by the heart or by the intake of air into the trachea.[1]:69 Air is taken in through spiracles along the sides of the abdomen and thorax supplying the trachea with oxygen as it goes through the lepidopteran's respiratory system. There are three different tracheae supplying oxygen diffusing oxygen throughout the species body: The dorsal, ventral, and visceral. The dorsal tracheae supply oxygen to the dorsal musculature and vessels, while the ventral tracheae supply the ventral musculature and nerve cord, and the visceral tracheae supply the guts, fat bodies, and gonads.[1]:71, 72
See also
| Wikispecies has information related to Insecta. |
| Wikimedia Commons has media related to Insect. |
| Wikiquote has quotations related to: Insects |
References
- Gullan, P.J.; P.S. Cranston (2005). The Insects: An Outline of Entomology (3 ed.). Oxford: Blackwell Publishing. ISBN 1-4051-1113-5.
- "O. Orkin Insect zoo". The University of Nebraska Department of Entomology. Archived from the original on 2009-06-02. Retrieved 2009-05-03.
- Resh, Vincent H.; Cardé, Ring T. (2009). Encyclopedia of Insects (2nd ed.). San DIego, CA: Academic Press. p. 12.
- Campbell, N. A. (1996) Biology (4th edition) Benjamin Cummings, New Work. p. 69 ISBN 0-8053-1957-3
- Gene Kritsky. (2002). A Survey of Entomology. iUniverse. ISBN 978-0-595-22143-1.
- "external morphology of Insects" (PDF). Archived from the original (PDF) on 2011-07-19. Retrieved 2011-03-20.
- Richards, O. W.; Davies, R.G. (1977). Imms' General Textbook of Entomology: Volume 1: Structure, Physiology and Development Volume 2: Classification and Biology. Berlin: Springer. ISBN 0-412-61390-5.
- Resh, Vincent H.; Ring T. Carde (July 1, 2009). Encyclopedia of Insects (2 ed.). U. S. A.: Academic Press. ISBN 978-0-12-374144-8.
- Smith, John Bernhard, Explanation of terms used in entomology Publisher: Brooklyn entomological society 1906 (May be downloaded from: https://archive.org/details/explanationofter00smit)
- Fox, Richard (6 Oct 2006). "External Anatomy". Lander University. Archived from the original on 2011-03-14. Retrieved 2011-03-20.
- "Archived copy" (PDF). Archived from the original (PDF) on 2008-10-01. Retrieved 2011-03-20. Cite journal requires
|journal=(help)CS1 maint: archived copy as title (link) - Meyer-Rochow, V.B. (1974). "Structure and function of the larval eye of the sawfly larva Perga". Journal of Insect Physiology. 20 (8): 1565–1591. doi:10.1016/0022-1910(74)90087-0. PMID 4854430.
- Völkel, R.; Eisner, M.; Weible, K.J. (2003). "Miniaturized imaging systems". Microelectronic Engineering. 67–68: 461–472. doi:10.1016/S0167-9317(03)00102-3. ISSN 0167-9317.
- Gaten, Edward (1998). "Optics and phylogeny: is there an insight? The evolution of superposition eyes in the Decapoda (Crustacea)". Contributions to Zoology. 67 (4): 223–236. doi:10.1163/18759866-06704001.
- Ritchie, Alexander (1985). "Ainiktozoon loganense Scourfield, a protochordate? from the Silurian of Scotland". Alcheringa. 9 (2): 137. doi:10.1080/03115518508618961.
- Mayer, G. (2006). "Structure and development of onychophoran eyes: What is the ancestral visual organ in arthropods?". Arthropod Structure and Development. 35 (4): 231–245. doi:10.1016/j.asd.2006.06.003. PMID 18089073.
- Chapman, R.F. (1998). The Insects: Structure and Function (4th ed.). Cambridge, UK: Cambridge University Press. ISBN 0521570484.
- Krause, A.F.; Winkler, A.; Dürr, V. (2013). "Central drive and proprioceptive control of antennal movements in the walking stick insect". Journal of Physiology, Paris. 107 (1–2): 116–129. doi:10.1016/j.jphysparis.2012.06.001. PMID 22728470. S2CID 11851224.
- Okada, J; Toh, Y (2001). "Peripheral representation of antennal orientation by the scapal hair plate of the cockroach Periplaneta americana". Journal of Experimental Biology. 204 (Pt 24): 4301–4309. PMID 11815654.
- Staudacher, E.; Gebhardt, M.J.; Dürr, V. (2005). "Antennal movements and mechanoreception: Neurobiology of active tactile sensors". Advances in Insect Physiology. 32: 49–205. doi:10.1016/S0065-2806(05)32002-9. ISBN 9780120242320.
- Servadei, A.; Zangheri, S.; Masutti, L. (1972). Entomologia generale ed applicata. CEDAM. pp. 492–530.
- Campbell, Frank L.; Priestly, June D. (1970). "Flagellar Annuli of Blattella germanica (Dictyoptera: Blattellidae).–Changes in Their Numbers and Dimensions during Postembryonic Development". Annals of the Entomological Society of America. 63: 81–88. doi:10.1093/aesa/63.1.81.
- "Insect antennae". The Amateur Entomologists' Society. Retrieved 2011-03-21.
- "Insect Morphology". University of Minisotta (Department of Entomology). Archived from the original on 2011-03-03. Retrieved 2011-03-21.
- Kirejtshuk, A.G. (November 2002). "Head". Beetles (Coleoptera) and coleopterologist. zin.ru. Retrieved 2011-03-21.
- Chapman, R.F. (1998). The Insects: Structure and function (4th ed.). Cambridge, New York: Cambridge University Press. ISBN 0-521-57048-4.
- Gilliott, Cedric (August 1995). Entomology (2 ed.). Springer-Verlag New York, LLC. ISBN 0-306-44967-6.
- Kapoor, V.C. C. (January 1998). Principles and Practices of Animal Taxonomy. 1 (1 ed.). Science Publishers. p. 48. ISBN 1-57808-024-X.
- "Mosquito biting mouthparts". allmosquitos.com. 2011. Retrieved April 17, 2011.
- Scoble, MJ. (1992). The Lepidoptera: Form, function, and diversity. Oxford Univ. Press. ISBN 978-1-4020-6242-1.
- Evans, Arthur V.; Bellamy, Charles (April 2000). An Inordinate Fondness for Beetles. ISBN 0-520-22323-3.
- Heppner, J. B. (2008). "Butterflies and moths". In Capinera, John L. (ed.). Encyclopedia of Entomology. Gale virtual reference library. 4 (2nd ed.). Springer Reference. p. 4345. ISBN 978-1-4020-6242-1.
- Agosta, Salvatore J.; Janzen, Daniel H. (2004). "Body size distributions of large Costa Rican dry forest moths and the underlying relationship between plant and pollinator morphology". Oikos. 108 (1): 183–193. doi:10.1111/j.0030-1299.2005.13504.x.
- Kunte, Krushnamegh (2007). "Allometry and functional constraints on proboscis lengths in butterflies" (PDF). Functional Ecology. 21 (5): 982–987. doi:10.1111/j.1365-2435.2007.01299.x. Archived from the original (PDF) on 29 June 2011. Retrieved 26 February 2011.
- "Sponging". University of Minissota. Archived from the original on September 27, 2011. Retrieved April 17, 2011.
- "Fly Mouthparts". School of Biological Sciences Online Learning Resources. University of Sydney. February 4, 2010. Retrieved April 17, 2011.
- Meyer, John R. (5 January 2007). "External Anatomy: WINGS". Department of Entomology, NC State University. Archived from the original on 16 July 2011. Retrieved 2011-03-21.
- Snodgrass, R. E. (December 1993). Principles of Insect Morphology. Cornell Univ Press. ISBN 0-8014-8125-2.
- Spieth, HT (1932). "A New Method of Studying the Wing Veins of the Mayflies and Some Results Therefrom (Ephemerida)" (PDF). Entomological News. Archived from the original (PDF) on 2011-09-30.
- "profemur, profemora - BugGuide.Net". bugguide.net. Retrieved 10 December 2016.
- "protibia - BugGuide.Net". bugguide.net. Retrieved 10 December 2016.
- Schneiderman, Howard A. (1960). "Discontinuous respiration in insects: role of the spiracles". Biol. Bull. 119 (3): 494–528. doi:10.2307/1539265. JSTOR 1539265.
- Eisemann, WK; Jorgensen, W. K.; Merritt, D. J.; Rice, M. J.; Cribb, B. W.; Webb, P. D.; Zalucki, M. P.; et al. (1984). "Do insects feel pain? — A biological view". Cellular and Molecular Life Sciences. 40 (2): 1420–1423. doi:10.1007/BF01963580. S2CID 3071.
- Tracey, J; Wilson, RI; Laurent, G; Benzer, S; et al. (18 April 2003). "painless, a Drosophila gene essential for nociception". Cell. 113 (2): 261–273. doi:10.1016/S0092-8674(03)00272-1. PMID 12705873. S2CID 1424315.
- Sømme, LS (14 January 2005). "Sentience and pain in invertebrates". Norwegian Scientific Committee for Food Safety. Retrieved September 30, 2009.
- "General Entomology - Digestive and Excritory system". NC state University. Retrieved 2009-05-03.
- Terra, Walter R.; Ferreira, Clélia (2009), "Digestive System", Encyclopedia of Insects, Elsevier, pp. 273–281, doi:10.1016/b978-0-12-374144-8.00083-7, ISBN 978-0-12-374144-8, retrieved 2020-09-29
- Terra, Walter R.; Ferreira, Clélia (2009-01-01), "Chapter 74 - Digestive System", in Resh, Vincent H.; Cardé, Ring T. (eds.), Encyclopedia of Insects (Second Edition), San Diego: Academic Press, pp. 273–281, doi:10.1016/b978-0-12-374144-8.00083-7, ISBN 978-0-12-374144-8, retrieved 2020-09-29
- Nation, James L. (November 2001). "15". Insect Physiology and Biochemistry (1 ed.). CRC Press. pp. 496pp. ISBN 0-8493-1181-0.
- Duncan, Carl D. (1939). A Contribution to The Biology of North American Vespine Wasps (1 ed.). Stanford: Stanford University Press. pp. 24–29.
- Meyer, John R. (17 February 2006). "Circulatory System". NC State University: Department of Entomology, NC State University. p. 1. Archived from the original on 27 September 2009. Retrieved 2009-10-11.
- Chown, S.L.; S.W. Nicholson (2004). Insect Physiological Ecology. New York: Oxford University Press. ISBN 0-19-851549-9.
- Richard W. Merritt, Kenneth W. Cummins, and Martin B. Berg (editors) (2007). An Introduction to the Aquatic Insects of North America (4th ed.). Kendall Hunt Publishers. ISBN 978-0-7575-5049-2.CS1 maint: multiple names: authors list (link) CS1 maint: extra text: authors list (link)
- Merritt, RW, KW Cummins, and MB Berg (2007). An Introduction To The Aquatic Insects Of North America. Kendall Hunt Publishing Company. ISBN 978-0-7575-4128-5.CS1 maint: multiple names: authors list (link)
- McGavin, G. C. (2001). Essential Entomology; An order by order introduction. New York: Oxford University Press. ISBN 9780198500025.
- Triplehorn, C. A.; Johnson, N. F. (2005). Borror and DeLong's Introduction to the Study of Insects. Brooks / Thomson Cole: Brooks / Thomson Cole.
- Elzinga, R.J. (2004). Fundamentals of Entomology (6th ed.). New Jersey USA: Pearson/Prentice Hall.
- Triplehorn, Charles A; Johnson, Norman F (2005). Borror and DeLong's introduction to the study of insects (7th ed.). Australia: Thomson, Brooks/Cole. ISBN 9780030968358.
- Gullan, P.J.; P.S. Cranston (2005). The Insects: An Outline of Entomology (3 ed.). Oxford: Blackwell Publishing. pp. 61–65. ISBN 1-4051-1113-5.
- De Carlo; J. A. (1983). "Hemipteros acuáticos y semiacuáticos. Estudio en grupos en las partes de igual función de los aparatos genitales masculinos de especies estudiadas". Revista de la Sociedad Entomológica Argentina. 42 (1/4): 149–154.
- Andersen, N. Møller (1991). "Marine insects: genital morphology, phylogeny and evolution of sea skaters, genus Halobates (Hemiptera: Gerridae)". Zoological Journal of the Linnean Society. 103 (1): 21–60. doi:10.1111/j.1096-3642.1991.tb00896.x.
- Eggleton, P (2001). "Termites and trees: a review of recent advances in termite phylogenetics". Insectes Sociaux. 48 (3): 187–193. doi:10.1007/pl00001766. S2CID 20011989.
- Lo, Nathan; Claudio Bandi; Hirofumi Watanabe; Christine Nalepa; Tiziana Beninat (2003). "Evidence for Cocladogenesis Between Diverse Dictyopteran Lineages and Their Intracellular Endosymbionts" (PDF). Molecular Biology and Evolution. 20 (6): 907–913. doi:10.1093/molbev/msg097. PMID 12716997.
- Leung, Chee Chee Leung (March 22, 2007). "Cave may hold missing link". theage.com.au. Retrieved 7 December 2013.
- Lo, N; Beninati, T; Stone, F; Walker, J; Sacchi, L (2007). "Cockroaches that lack Blattabacterium endosymbionts: the phylogenetically divergent genus Nocticola". Biology Letters. 3 (3): 327–30. doi:10.1098/rsbl.2006.0614. PMC 2464682. PMID 17376757.
- Kunkel, Joseph G. "How do cockroaches breathe?". The Cockroach FAQ. UMass Amherst. Retrieved 7 December 2013.
- Gillot, C. (1995). "Butterflies and moths". Entomology (2 ed.). pp. 246–266. ISBN 978-0-306-44967-3. Retrieved 14 November 2010.
- Schmidt-Nielsen, Knut (Jan 15, 1997). "Insect Respiration". Animal Physiology: Adaptation and Environment (5 (illustrated) ed.). Cambridge University Press. p. 55. ISBN 0-521-57098-0. Retrieved 6 March 2010.
- Hoell, H.V., Doyen, J.T. & Purcell, A.H. (1998). Introduction to Insect Biology and Diversity, 2nd ed. Oxford University Press. pp. 493–499. ISBN 0-19-510033-6.CS1 maint: multiple names: authors list (link)
- Williams, C. M. (1947). "Physiology of insect diapause. II. Interaction between the pupal brain and prothoracic glands in the metamorphosis of the giant silkworm "Platysamia cecropia"". Biol. Bull. 92 (2): 89–180. doi:10.2307/1538279. JSTOR 1538279. PMID 20268135.
- Lighton, J. R. B.; Lovegrove, B. G. (1990). "A temperature-induced switch from diffusive to convective ventilation in the honeybee". Journal of Experimental Biology. 154: 509–516.









.jpg.webp)

.jpg.webp)