Interleukin 10
Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti-inflammatory cytokine. In humans, interleukin 10 is encoded by the IL10 gene.[5] IL-10 signals through a receptor complex consisting of two IL-10 receptor-1 and two IL-10 receptor-2 proteins.[6] Consequently, the functional receptor consists of four IL-10 receptor molecules. IL-10 binding induces STAT3 signalling via the phosphorylation of the cytoplasmic tails of IL-10 receptor 1 + IL-10 receptor 2 by JAK1 and Tyk2 respectively.[6]
Gene and protein structure
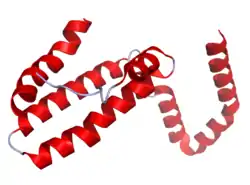
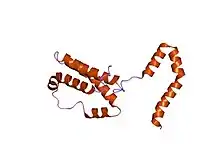
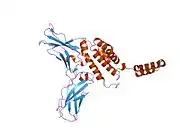
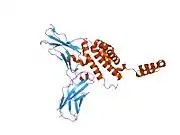
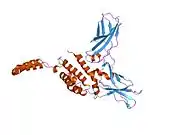
The IL-10 protein is a homodimer; each of its subunits is 178-amino-acid long.[7]
IL-10 is classified as a class-2 cytokine, a set of cytokines including IL-19, IL-20, IL-22, IL-24 (Mda-7), IL-26 and interferons type-I (IFN-alpha, -beta, -epsilon, -kappa, -omega), type-II (IFN-gamma) and type-III (IFN-lambda,[8] also known as IL-28A, IL-28B, and IL-29).[9]
Expression and synthesis
In humans, IL-10 is encoded by the IL10 gene, which is located on chromosome 1 and comprises 5 exons,[5] and is primarily produced by monocytes and, to a lesser extent, lymphocytes, namely type-II T helper cells (TH2), mast cells, CD4+CD25+Foxp3+ regulatory T cells, and in a certain subset of activated T cells and B cells. IL-10 can be produced by monocytes upon PD-1 triggering in these cells.[10] IL-10 upregulation is also mediated by GPCRs, such as beta-2 adrenergic[11] and type 2 cannabinoid[12] receptors. The expression of IL-10 is minimal in unstimulated tissues and seems to require triggering by commensal or pathogenic flora.[13] IL-10 expression is tightly regulated at the transcriptional and post-transcriptional level. Extensive IL-10 locus remodeling is observed in monocytes upon stimulation of TLR or Fc receptor pathways.[14] IL-10 induction involves ERK1/2, p38 and NF-κB signalling and transcriptional activation via promoter binding of the transcription factors NF-κB and AP-1.[14] IL-10 may autoregulate its expression via a negative feed-back loop involving autocrine stimulation of the IL-10 receptor and inhibition of the p38 signaling pathway.[15] Additionally, IL-10 expression is extensively regulated at the post-transcriptional level, which may involve control of mRNA stability via AU-rich elements[16] and by microRNAs such as let-7[17] or miR-106.[18]
Function
IL-10 is a cytokine with multiple, pleiotropic, effects in immunoregulation and inflammation. It downregulates the expression of Th1 cytokines, MHC class II antigens, and co-stimulatory molecules on macrophages. It also enhances B cell survival, proliferation, and antibody production. IL-10 can block NF-κB activity, and is involved in the regulation of the JAK-STAT signaling pathway.
Discovered in 1991[19] IL-10 was initially reported to suppress cytokine secretion, antigen presentation and CD4+ T cell activation.[20][21][22][23] Further investigation has shown that IL-10 predominantly inhibits lipopolysaccharide (LPS) and bacterial product mediated induction of the pro-inflammatory cytokines TNFα,[24] IL-1β,[24] IL-12,[25] and IFNγ[26] secretion from Toll-Like Receptor (TLR) triggered myeloid lineage cells.
Effect on tumors
Over time a more nuanced picture of IL-10's function has emerged as treatment of tumor bearing mice has been shown to inhibit tumor metastasis.[27] Additional investigation by multiple laboratories has generated data that further supports IL-10's immunostimulatory capacity in an immunoncology context. Expression of IL-10 from transfected tumor cell lines[28][29] in IL-10 transgenic mice[30] or dosing with IL-10 leads to control of primary tumor growth and decreased metastatic burden.[31][32] More recently, PEGylated recombinant murine IL-10 (PEG-rMuIL-10) has been shown to induce IFNγ and CD8+ T cell dependent anti-tumor immunity.[33][34] More specifically, PEGylated recombinant human IL-10 (PEG-rHuIL-10) has been shown to enhance CD8+ T cell secretion of the cytotoxic molecules Granzyme B and Perforin and potentiate T cell receptor dependent IFNγ secretion.[35]
Role in disease
A study in mice has shown that IL-10 is also produced by mast cells, counteracting the inflammatory effect that these cells have at the site of an allergic reaction.[36]
IL-10 is capable of inhibiting synthesis of pro-inflammatory cytokines such as IFN-γ, IL-2, IL-3, TNFα and GM-CSF made by cells such as macrophages and Th1 T cells. It also displays a potent ability to suppress the antigen-presentation capacity of antigen presenting cells; however, it is also stimulatory towards certain T cells (Th2) and mast cells and stimulates B cell maturation and antibody production.
IL-10 checks the inducible form of Cyclo-oxygenase, Cyclo-oxygenase-2 (COX-2). Lack of IL-10 has been shown to cause COX activation and resultant Thromboxane receptor activation to cause vascular endothelial and cardiac dysfunctions in mice. Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age.[37]
IL-10 is linked to the myokines, as exercise provokes an increase in circulating levels of IL-1ra, IL-10, and sTNF-R, suggesting that physical exercise fosters an environment of anti-inflammatory cytokines.[38][39]
Lower levels of IL-10 have been observed in individuals diagnosed with multiple sclerosis when compared to healthy individuals.[40] Due to a decrease in IL-10 levels, TNFα levels are not regulated effectively as IL-10 regulates the TNF-α-converting enzyme.[41] As a result, TNFα levels rise and result in inflammation.[42] TNFα itself induces demyelination of the oliodendroglial via TNF receptor 1, while chronic inflammation has been linked to demyelination of neurons.[42]
In melanoma cell lines, IL-10 modulates the surface expression of NKG2D ligands.[43]
Clinical use or trials
Knockout studies in mice suggested the function of this cytokine as an essential immunoregulator in the intestinal tract.[44] and, indeed, patients with Crohn's disease react favorably towards treatment with recombinant interleukin-10-producing bacteria, demonstrating the importance of IL-10 for counteracting the hyperactive immune response in the human body.[45]
Due to the data, thousands of patients suffering from a variety of autoimmune diseases were treated with recombinant human IL-10 (rHuIL-10) in clinical trials. Contrary to expectations, rHuIL-10 treatment did not significantly impact disease in patients with Crohn's disease.[46][47][48] or rheumatoid arthritis.[49] rHuIL-10 treatment initially exhibited promising clinical data in psoriasis.[50] but failed to achieve clinical significance in a randomized, double blind, placebo controlled Phase II trial.[51] Further investigation of rHuIL-10's effects in humans suggests that rather than inhibiting inflammation, rHuIL-10 is capable of exerting pro-inflammatory effects.[52][53]
PEGylated forms
Further to these data, a Phase I immunoncology clinical trial is currently being conducted to assess the therapeutic capacity of PEGylated recombinant human IL-10 (PEG-rHuIL-10, AM0010).[54] Consistent with preclinical immunoncology data, investigators report substantial anti-tumor efficacy.[54] Contrary to the reported immunosuppressive effects of IL-10 generated in vitro and in vivo,[21][22][23][24][25] treatment of cancer patients with PEG-rHuIL-10 elicits a dose titratable induction of the immune stimulatory cytokines IFNγ, IL-18, IL-7, GM-CSF and IL-4.[54] Furthermore, treated patients exhibit fold increases of peripheral CD8+ T cells expressing markers of activation, such as programmed death 1 (PD1)+, lymphocyte activation gene 3 (LAG3)+ and increased Fas Ligand (FasL) and a decrease in serum TGFβ.[54] These findings are consistent with the published preclinical immunoncology reports using PEG-rMuIL-10[33][34] and with previous findings treating humans with rHuIL-10.[52][53] These data suggest that while IL-10 can exert immunosuppressive effects in context of bacterial product stimulated myeloid cells, rHuIL-10/PEG-rHuIL-10 treatment of humans is predominantly immunostimulatory. As of 2018 AM0010 (aka pegilodecakin) is in phase 3 clinical trials.[55]
Interactions
IL-10 has been shown to interact with Interleukin 10 receptor, alpha subunit.[56][57][58][59][60]
The receptor complex for IL-10 also requires the IL10R2 chain to initiate signalling. This ligand–receptor combination is found in birds and frogs, and is also likely to exist in bony fish.
References
- GRCh38: Ensembl release 89: ENSG00000136634 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000016529 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Eskdale J, Kube D, Tesch H, Gallagher G (1997). "Mapping of the human IL10 gene and further characterization of the 5' flanking sequence". Immunogenetics. 46 (2): 120–8. doi:10.1007/s002510050250. PMID 9162098.
- Mosser DM, Zhang X (December 2008). "Interleukin-10: new perspectives on an old cytokine". Immunological Reviews. 226 (1): 205–18. doi:10.1111/j.1600-065X.2008.00706.x. PMC 2724982. PMID 19161426.
- Zdanov A, Schalk-Hihi C, Gustchina A, Tsang M, Weatherbee J, Wlodawer A (June 1995). "Crystal structure of interleukin-10 reveals the functional dimer with an unexpected topological similarity to interferon gamma". Structure. 3 (6): 591–601. doi:10.1016/S0969-2126(01)00193-9. PMID 8590020.
- Lazear HM, Nice TJ, Diamond MS (July 2015). "Interferon-λ: Immune Functions at Barrier Surfaces and Beyond". Immunity. 43 (1): 15–28. doi:10.1016/j.immuni.2015.07.001. PMC 4527169. PMID 26200010.
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB (2004). "Interleukin-10 and related cytokines and receptors". Annual Review of Immunology. 22 (1): 929–79. doi:10.1146/annurev.immunol.22.012703.104622. PMID 15032600.
- Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP (April 2010). "Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection". Nature Medicine. 16 (4): 452–9. doi:10.1038/nm.2106. PMC 4229134. PMID 20208540.
- Ağaç, Didem; Estrada, Leonardo D.; Maples, Robert; Hooper, Lora V.; Farrar, J. David (November 2018). "The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion". Brain, Behavior, and Immunity. 74: 176–185. doi:10.1016/j.bbi.2018.09.004. ISSN 1090-2139. PMC 6289674. PMID 30195028.
- Saroz, Yurii; Kho, Dan T.; Glass, Michelle; Graham, Euan Scott; Grimsey, Natasha Lillia (2019-10-19). "Cannabinoid Receptor 2 (CB 2 ) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes". ACS Pharmacology & Translational Science. 2 (6): 414–428. doi:10.1021/acsptsci.9b00049. ISSN 2575-9108.
- Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF (March 2012). "IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines". PLOS ONE. 7 (3): e33628. doi:10.1371/journal.pone.0033628. PMC 3306427. PMID 22438968.
- Saraiva M, O'Garra A (March 2010). "The regulation of IL-10 production by immune cells". Nature Reviews. Immunology. 10 (3): 170–81. doi:10.1038/nri2711. hdl:1822/29592. PMID 20154735.
- Hammer M, Mages J, Dietrich H, Schmitz F, Striebel F, Murray PJ, Wagner H, Lang R (October 2005). "Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10". European Journal of Immunology. 35 (10): 2991–3001. doi:10.1002/eji.200526192. PMID 16184516.
- Powell MJ, Thompson SA, Tone Y, Waldmann H, Tone M (July 2000). "Posttranscriptional regulation of IL-10 gene expression through sequences in the 3'-untranslated region". Journal of Immunology. 165 (1): 292–6. doi:10.4049/jimmunol.165.1.292. PMID 10861064.
- Schulte LN, Eulalio A, Mollenkopf HJ, Reinhardt R, Vogel J (May 2011). "Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family". The EMBO Journal. 30 (10): 1977–89. doi:10.1038/emboj.2011.94. PMC 3098495. PMID 21468030.
- Sharma A, Kumar M, Aich J, Hariharan M, Brahmachari SK, Agrawal A, Ghosh B (April 2009). "Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a". Proceedings of the National Academy of Sciences of the United States of America. 106 (14): 5761–6. doi:10.1073/pnas.0808743106. PMC 2659714. PMID 19307576.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001-01-01). "Interleukin-10 and the interleukin-10 receptor". Annual Review of Immunology. 19 (1): 683–765. doi:10.1146/annurev.immunol.19.1.683. PMID 11244051.
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (November 1991). "Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes". The Journal of Experimental Medicine. 174 (5): 1209–20. doi:10.1084/jem.174.5.1209. PMC 2119001. PMID 1940799.
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE (October 1991). "Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression". The Journal of Experimental Medicine. 174 (4): 915–24. doi:10.1084/jem.174.4.915. PMC 2118975. PMID 1655948.
- Akdis CA, Joss A, Akdis M, Faith A, Blaser K (September 2000). "A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding". FASEB Journal. 14 (12): 1666–8. doi:10.1096/fj.99-0874fje. PMID 10973911.
- Joss A, Akdis M, Faith A, Blaser K, Akdis CA (June 2000). "IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway". European Journal of Immunology. 30 (6): 1683–90. doi:10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. PMID 10898505.
- Opp MR, Smith EM, Hughes TK (July 1995). "Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep". Journal of Neuroimmunology. 60 (1–2): 165–8. doi:10.1016/0165-5728(95)00066-b. PMID 7642744.
- Aste-Amezaga M, Ma X, Sartori A, Trinchieri G (June 1998). "Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10". Journal of Immunology. 160 (12): 5936–44. PMID 9637507.
- Varma TK, Toliver-Kinsky TE, Lin CY, Koutrouvelis AP, Nichols JE, Sherwood ER (September 2001). "Cellular mechanisms that cause suppressed gamma interferon secretion in endotoxin-tolerant mice". Infection and Immunity. 69 (9): 5249–63. doi:10.1128/iai.69.9.5249-5263.2001. PMC 98633. PMID 11500393.
- Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, Rong H, Chen J, Wang XY, Catino JJ, King I (August 1996). "Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism". The Journal of Experimental Medicine. 184 (2): 579–84. doi:10.1084/jem.184.2.579. PMC 2192723. PMID 8760811.
- Sun H, Jackson MJ, Kundu N, Fulton AM (February 1999). "Interleukin-10 gene transfer activates interferon-gamma and the interferon-gamma-inducible genes Gbp-1/Mag-1 and Mig-1 in mammary tumors". International Journal of Cancer. 80 (4): 624–9. doi:10.1002/(sici)1097-0215(19990209)80:4<624::aid-ijc23>3.0.co;2-9. PMID 9935167.
- Sun H, Gutierrez P, Jackson MJ, Kundu N, Fulton AM (2000-04-01). "Essential role of nitric oxide and interferon-gamma for tumor immunotherapy with interleukin-10". Journal of Immunotherapy. 23 (2): 208–14. doi:10.1097/00002371-200003000-00005. PMID 10746547.
- Groux H, Cottrez F, Rouleau M, Mauze S, Antonenko S, Hurst S, McNeil T, Bigler M, Roncarolo MG, Coffman RL (February 1999). "A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells". Journal of Immunology. 162 (3): 1723–9. PMID 9973435.
- Fujii S, Shimizu K, Shimizu T, Lotze MT (October 2001). "Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ". Blood. 98 (7): 2143–51. doi:10.1182/blood.v98.7.2143. PMID 11568001.
- Berman RM, Suzuki T, Tahara H, Robbins PD, Narula SK, Lotze MT (July 1996). "Systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice". Journal of Immunology. 157 (1): 231–8. PMID 8683120.
- Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, Gorman DM, Oft M (July 2012). "IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs". Cancer Research. 72 (14): 3570–81. doi:10.1158/0008-5472.CAN-12-0721. PMID 22581824.
- Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, Sheppard C, Hong K, Cutler C, Turner S, LaFace D, Kleinschek M, Judo M, Ayanoglu G, Langowski J, Gu D, Paporello B, Murphy E, Sriram V, Naravula S, Desai B, Medicherla S, Seghezzi W, McClanahan T, Cannon-Carlson S, Beebe AM, Oft M (December 2011). "IL-10 elicits IFNγ-dependent tumor immune surveillance". Cancer Cell. 20 (6): 781–96. doi:10.1016/j.ccr.2011.11.003. PMID 22172723.
- Chan IH, Wu V, Bilardello M, Mar E, Oft M, Van Vlasselaer P, Mumm JB (December 2015). "The Potentiation of IFN-γ and Induction of Cytotoxic Proteins by Pegylated IL-10 in Human CD8 T Cells". Journal of Interferon & Cytokine Research. 35 (12): 948–55. doi:10.1089/jir.2014.0221. PMID 26309093.
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ (October 2007). "Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B". Nature Immunology. 8 (10): 1095–104. doi:10.1038/ni1503. PMID 17767162.
- Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, Fraser CD, Ellis C, Ross D, Vandegaer K, Bedja D, Gabrielson K, Walston JD, Berkowitz DE, Barouch LA (February 2013). "Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age". Experimental Gerontology. 48 (2): 128–35. doi:10.1016/j.exger.2012.11.001. PMC 3744178. PMID 23159957.
- Ostrowski K, Schjerling P, Pedersen BK (December 2000). "Physical activity and plasma interleukin-6 in humans--effect of intensity of exercise". European Journal of Applied Physiology. 83 (6): 512–5. doi:10.1007/s004210000312. PMID 11192058.
- Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (February 1999). "Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans". The Journal of Physiology. 515 (1): 287–91. doi:10.1111/j.1469-7793.1999.287ad.x. PMC 2269132. PMID 9925898.
- Ozenci V, Kouwenhoven M, Huang YM, Xiao B, Kivisäkk P, Fredrikson S, Link H (May 1999). "Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment". Scandinavian Journal of Immunology. 49 (5): 554–61. doi:10.1046/j.1365-3083.1999.00546.x. PMID 10320650.
- Brennan FM, Green P, Amjadi P, Robertshaw HJ, Alvarez-Iglesias M, Takata M (April 2008). "Interleukin-10 regulates TNF-alpha-converting enzyme (TACE/ADAM-17) involving a TIMP-3 dependent and independent mechanism". European Journal of Immunology. 38 (4): 1106–17. doi:10.1002/eji.200737821. PMID 18383040.
- Nakahara J, Maeda M, Aiso S, Suzuki N (February 2012). "Current concepts in multiple sclerosis: autoimmunity versus oligodendrogliopathy". Clinical Reviews in Allergy & Immunology. 42 (1): 26–34. doi:10.1007/s12016-011-8287-6. PMID 22189514.
- Serrano AE, Menares-Castillo E, Garrido-Tapia M, Ribeiro CH, Hernández CJ, Mendoza-Naranjo A, Gatica-Andrades M, Valenzuela-Diaz R, Zúñiga R, López MN, Salazar-Onfray F, Aguillón JC, Molina MC (March 2011). "Interleukin 10 decreases MICA expression on melanoma cell surface". Immunology and Cell Biology. 89 (3): 447–57. doi:10.1038/icb.2010.100. PMID 20714339.
- "Entrez Gene: IL10 interleukin 10".
- Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L (June 2006). "A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease". Clinical Gastroenterology and Hepatology. 4 (6): 754–9. doi:10.1016/j.cgh.2006.03.028. PMID 16716759.
- Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A, Feagan B (December 2000). "Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group". Gastroenterology. 119 (6): 1473–82. doi:10.1053/gast.2000.20229. PMID 11113068.
- Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P, Isaacs K, van Deventer SJ, Koningsberger JC, Cohard M, LeBeaut A, Hanauer SB (December 2000). "Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Crohn's Disease IL-10 Cooperative Study Group". Gastroenterology. 119 (6): 1461–72. doi:10.1053/gast.2000.20196. PMID 11113067.
- van Deventer SJ, Elson CO, Fedorak RN (August 1997). "Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group". Gastroenterology. 113 (2): 383–9. doi:10.1053/gast.1997.v113.pm9247454. PMID 9247454.
- van Roon J, Wijngaarden S, Lafeber FP, Damen C, van de Winkel J, Bijlsma JW (April 2003). "Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation". The Journal of Rheumatology. 30 (4): 648–51. PMID 12672180.
- Asadullah K, Döcke WD, Ebeling M, Friedrich M, Belbe G, Audring H, Volk HD, Sterry W (February 1999). "Interleukin 10 treatment of psoriasis: clinical results of a phase 2 trial". Archives of Dermatology. 135 (2): 187–92. doi:10.1001/archderm.135.2.187. PMID 10052405.
- Kimball AB, Kawamura T, Tejura K, Boss C, Hancox AR, Vogel JC, Steinberg SM, Turner ML, Blauvelt A (October 2002). "Clinical and immunologic assessment of patients with psoriasis in a randomized, double-blind, placebo-controlled trial using recombinant human interleukin 10". Archives of Dermatology. 138 (10): 1341–6. doi:10.1001/archderm.138.10.1341. PMID 12374540.
- Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T (September 2000). "Proinflammatory effects of IL-10 during human endotoxemia". Journal of Immunology. 165 (5): 2783–9. doi:10.4049/jimmunol.165.5.2783. PMID 10946310.
- Tilg H, van Montfrans C, van den Ende A, Kaser A, van Deventer SJ, Schreiber S, Gregor M, Ludwiczek O, Rutgeerts P, Gasche C, Koningsberger JC, Abreu L, Kuhn I, Cohard M, LeBeaut A, Grint P, Weiss G (February 2002). "Treatment of Crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma". Gut. 50 (2): 191–5. doi:10.1136/gut.50.2.191. PMC 1773093. PMID 11788558.
- Infante JR, Naing A, Papadopoulos KP, Autio KA, Ott PA, Wong DJ, Falchook GS, Patel MR, Pant S (2015-05-20). "A first-in-human dose escalation study of PEGylated recombinant human IL-10 (AM0010) in advanced solid tumors". ASCO Meeting Abstracts. 33 (15_suppl): 3017.
- Early Data Supports Phase 3 Trial of Pegilodecakin as Possible Treatment for Advanced Pancreatic Cancer
- Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW (December 1993). "A receptor for interleukin 10 is related to interferon receptors". Proceedings of the National Academy of Sciences of the United States of America. 90 (23): 11267–71. doi:10.1073/pnas.90.23.11267. PMC 47963. PMID 8248239.
- Josephson K, Logsdon NJ, Walter MR (July 2001). "Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site". Immunity. 15 (1): 35–46. doi:10.1016/S1074-7613(01)00169-8. PMID 11485736.
- Tan JC, Braun S, Rong H, DiGiacomo R, Dolphin E, Baldwin S, Narula SK, Zavodny PJ, Chou CC (May 1995). "Characterization of recombinant extracellular domain of human interleukin-10 receptor". The Journal of Biological Chemistry. 270 (21): 12906–11. doi:10.1074/jbc.270.21.12906. PMID 7759550.
- Josephson K, McPherson DT, Walter MR (December 2001). "Purification, crystallization and preliminary X-ray diffraction of a complex between IL-10 and soluble IL-10R1". Acta Crystallographica Section D. 57 (Pt 12): 1908–11. doi:10.1107/S0907444901016249. PMID 11717514.
- Hoover DM, Schalk-Hihi C, Chou CC, Menon S, Wlodawer A, Zdanov A (May 1999). "Purification of receptor complexes of interleukin-10 stoichiometry and the importance of deglycosylation in their crystallization". European Journal of Biochemistry. 262 (1): 134–41. doi:10.1046/j.1432-1327.1999.00363.x. PMID 10231374.
Further reading
- Bortesi L, Rossato M, Schuster F, Raven N, Stadlmann J, Avesani L, Falorni A, Bazzoni F, Bock R, Schillberg S, Pezzotti M (March 2009). "Viral and murine interleukin-10 are correctly processed and retain their biological activity when produced in tobacco". BMC Biotechnology. 9 (1): 22. doi:10.1186/1472-6750-9-22. PMC 2667500. PMID 19298643.
- Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, Che F, Chen X, Ren H, Hong M, Wang J (March 2019). "Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage". Prog. Neurobiol. 178: 101610. doi:10.1016/j.pneurobio.2019.03.003. PMID 30923023.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001). "Interleukin-10 and the interleukin-10 receptor". Annual Review of Immunology. 19 (1): 683–765. doi:10.1146/annurev.immunol.19.1.683. PMID 11244051.
- Girndt M (2003). "Humoral immune responses in uremia and the role of IL-10". Blood Purification. 20 (5): 485–8. doi:10.1159/000063553. PMID 12207099.
- Beebe AM, Cua DJ, de Waal Malefyt R (2003). "The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS)". Cytokine & Growth Factor Reviews. 13 (4–5): 403–12. doi:10.1016/S1359-6101(02)00025-4. PMID 12220553.
- Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM (January 2003). "The dual role of IL-10". Trends in Immunology. 24 (1): 36–43. doi:10.1016/S1471-4906(02)00009-1. PMID 12495723.
- Roncarolo MG, Battaglia M, Gregori S (June 2003). "The role of interleukin 10 in the control of autoimmunity". Journal of Autoimmunity. 20 (4): 269–72. doi:10.1016/S0896-8411(03)00047-7. PMID 12791310.
- Groux H, Cottrez F (June 2003). "The complex role of interleukin-10 in autoimmunity". Journal of Autoimmunity. 20 (4): 281–5. doi:10.1016/S0896-8411(03)00044-1. PMID 12791313.
- Llorente L, Richaud-Patin Y (June 2003). "The role of interleukin-10 in systemic lupus erythematosus". Journal of Autoimmunity. 20 (4): 287–9. doi:10.1016/S0896-8411(03)00043-X. PMID 12791314.
- Asadullah K, Sabat R, Friedrich M, Volk HD, Sterry W (June 2004). "Interleukin-10: an important immunoregulatory cytokine with major impact on psoriasis". Current Drug Targets. Inflammation and Allergy. 3 (2): 185–92. doi:10.2174/1568010043343886. PMID 15180472.
- Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M (April 2005). "IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly". Kidney International. 67 (4): 1216–33. doi:10.1111/j.1523-1755.2005.00200.x. PMID 15780075.
- Chang CF, Wan J, Li Q, Renfroe SC, Heller NM, Wang J (July 2017). "Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage". Neurobiol. Dis. 103: 54–69. doi:10.1016/j.nbd.2017.03.016. PMC 5540140. PMID 28365213.
- Copeland KF (December 2005). "Modulation of HIV-1 transcription by cytokines and chemokines". Mini Reviews in Medicinal Chemistry. 5 (12): 1093–101. doi:10.2174/138955705774933383. PMID 16375755.
External links
 Media related to Interferons or interleukin-10 (IL-10) at Wikimedia Commons
Media related to Interferons or interleukin-10 (IL-10) at Wikimedia Commons- Interleukin-10 at the US National Library of Medicine Medical Subject Headings (MeSH)