Millipede
Millipedes are a group of arthropods that are characterised by having two pairs of jointed legs on most body segments; they are known scientifically as the class Diplopoda, the name being derived from this feature. Each double-legged segment is a result of two single segments fused together. Most millipedes have very elongated cylindrical or flattened bodies with more than 20 segments, while pill millipedes are shorter and can roll into a ball. Although the name "millipede" derives from the Latin for "thousand feet", no known species has 1,000; the record of 750 legs belongs to Illacme plenipes. There are approximately 12,000 named species classified into 16 orders and around 140 families, making Diplopoda the largest class of myriapods, an arthropod group which also includes centipedes and other multi-legged creatures.
| Millipedes | |
|---|---|
 | |
| An assortment of millipedes (not to scale) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Myriapoda |
| Class: | Diplopoda Blainville in Gervais, 1844 |
| Subclasses | |
| |
| Diversity | |
| 16 orders, c. 12,000 species | |
Most millipedes are slow-moving detritivores, eating decaying leaves and other dead plant matter. Some eat fungi or suck plant fluids, and a small minority are predatory. Millipedes are generally harmless to humans, although some can become household or garden pests. Millipedes can be unwanted especially in greenhouses where they can cause severe damage to emergent seedlings. Most millipedes defend themselves with a variety of chemicals secreted from pores along the body, although the tiny bristle millipedes are covered with tufts of detachable bristles. Its primary defence mechanism is to curl into a tight coil, thereby protecting its legs and other vital delicate areas on the body behind a hard exoskeleton. Reproduction in most species is carried out by modified male legs called gonopods, which transfer packets of sperm to females.
First appearing in the Silurian period, millipedes are some of the oldest known land animals. Some members of prehistoric groups grew to over 2 m (6 ft 7 in); the largest modern species reach maximum lengths of 27 to 38 cm (11 to 15 in). The longest extant species is the giant African millipede (Archispirostreptus gigas).
Among myriapods, millipedes have traditionally been considered most closely related to the tiny pauropods, although some molecular studies challenge this relationship. Millipedes can be distinguished from the somewhat similar but only distantly related centipedes (class Chilopoda), which move rapidly, are venomous, carnivorous, and have only a single pair of legs on each body segment. The scientific study of millipedes is known as diplopodology, and a scientist who studies them is called a diplopodologist.
Etymology and names
The term "millipede" is widespread in popular and scientific literature, but among North American scientists, the term "milliped" (without the terminal e) is also used.[1] Other vernacular names include "thousand-legger" or simply "diplopod".[2] The science of millipede biology and taxonomy is called diplopodology: the study of diplopods.
Classification
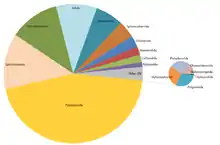
Approximately 12,000 millipede species have been described. Estimates of the true number of species on earth range from 15,000[4] to as high as 80,000.[5] Few species of millipede are at all widespread; they have very poor dispersal abilities, depending as they do on terrestrial locomotion and humid habitats. These factors have favoured genetic isolation and rapid speciation, producing many lineages with restricted ranges.[6]
The living members of the Diplopoda are divided into sixteen orders in two subclasses.[3] The basal subclass Penicillata contains a single order, Polyxenida (bristle millipedes). All other millipedes belong to the subclass Chilognatha consisting of two infraclasses: Pentazonia, containing the short-bodied pill millipedes, and Helminthomorpha (worm-like millipedes), containing the great majority of the species.[7][8]
Outline of classification
The higher-level classification of millipedes is presented below, based on Shear, 2011,[3] and Shear & Edgecombe, 2010[9] (extinct groups). Recent cladistic and molecular studies have challenged the traditional classification schemes above, and in particular the position of the orders Siphoniulida and Polyzoniida is not yet well established.[5] The placement and positions of extinct groups (†) known only from fossils is tentative and not fully resolved.[5][9] After each name is listed the author citation: the name of the person who coined the name or defined the group, even if not at the current rank.
Class Diplopoda de Blainville in Gervais, 1844
- Subclass Penicillata Latreille, 1831
- Order Polyxenida Verhoeff, 1934
- Subclass †Arthropleuridea (placed in Penicillata by some authors)[9]
- Order †Arthropleurida Waterlot, 1934
- Order †Eoarthropleurida Shear & Selden, 1995
- Order †Microdecemplicida Wilson & Shear, 2000
- Subclass Chilognatha Latrielle, 1802
- Order †Zosterogrammida Wilson, 2005 (Chilognatha incertae sedis)[9]
- Infraclass Pentazonia Brandt, 1833
- Order †Amynilyspedida Hoffman, 1969
- Superorder Limacomorpha Pocock, 1894
- Order Glomeridesmida Cook, 1895
- Superorder Oniscomorpha Pocock, 1887
- Order Glomerida Brandt, 1833
- Order Sphaerotheriida Brandt, 1833
- Infraclass Helminthomorpha Pocock, 1887
- Superorder †Archipolypoda Scudder, 1882
- Order †Archidesmida Wilson & Anderson 2004
- Order †Cowiedesmida Wilson & Anderson 2004
- Order †Euphoberiida Hoffman, 1969
- Order †Palaeosomatida Hannibal & Krzeminski, 2005
- Order †Pleurojulida Schneider & Werneburg, 1998 (possibly sister to Colobognatha)[5]
- Subterclass Colobognatha Brandt, 1834
- Order Platydesmida Cook, 1895
- Order Polyzoniida Cook, 1895
- Order Siphonocryptida Cook, 1895
- Order Siphonophorida Newport, 1844
- Subterclass Eugnatha Attems, 1898
- Superorder Juliformia Attems, 1926
- Order Julida Brandt, 1833
- Order Spirobolida Cook, 1895
- Order Spirostreptida Brandt, 1833
- Superfamily †Xyloiuloidea Cook, 1895 (Sometimes aligned with Spirobolida)[10]
- Superorder Nematophora Verhoeff, 1913
- Order Callipodida Pocock, 1894
- Order Chordeumatida Pocock 1894
- Order Stemmiulida Cook, 1895
- Order Siphoniulida Cook, 1895
- Superorder Merocheta Cook, 1895
- Order Polydesmida Pocock, 1887
- Superorder Juliformia Attems, 1926
- Superorder †Archipolypoda Scudder, 1882
Evolution
Millipedes are among the first animals to have colonised land during the Silurian period.[11] Early forms probably ate mosses and primitive vascular plants. There are two major groups of millipedes whose members are all extinct: the Archipolypoda ("ancient, many-legged ones") which contain the oldest known terrestrial animals, and Arthropleuridea, which contain the largest known land invertebrates. The earliest known land creature, Pneumodesmus newmani, was a 1 cm (0.4 in) long archipolypodan that lived 428 million years ago in the upper Silurian, and has clear evidence of spiracles (breathing holes) attesting to its air-breathing habits.[9][12] During the Upper Carboniferous (340 to 280 million years ago), Arthropleura became the largest known land-dwelling invertebrate on record, reaching lengths of at least 2 m (6 ft 7 in).[13] Millipedes also exhibit the earliest evidence of chemical defence, as some Devonian fossils have defensive gland openings called ozopores.[9] Millipedes, centipedes, and other terrestrial arthropods attained very large sizes in comparison to modern species in the oxygen-rich environments of the Devonian and Carboniferous periods, and some could grow larger than one metre. As oxygen levels lowered through time, arthropods became smaller.[14]
Living groups
_(3405605943).jpg.webp)
The history of scientific millipede classification began with Carl Linnaeus, who in his 10th edition of Systema Naturae, 1758, named seven species of Julus as "Insecta Aptera" (wingless insects).[15] In 1802, the French zoologist Pierre André Latreille proposed the name Chilognatha as the first group of what are now the Diplopoda, and in 1840 the German naturalist Johann Friedrich von Brandt produced the first detailed classification. The name Diplopoda itself was coined in 1844 by the French zoologist Henri Marie Ducrotay de Blainville. From 1890 to 1940, millipede taxonomy was driven by relatively few researchers at any given time, with major contributions by Carl Attems, Karl Wilhelm Verhoeff and Ralph Vary Chamberlin, who each described over 1,000 species, as well as Orator F. Cook, Filippo Silvestri, R. I. Pocock, and Henry W. Brölemann.[5] This was a period when the science of diplopodology flourished: rates of species descriptions were on average the highest in history, sometimes exceeding 300 per year.[4]
In 1971, the Dutch biologist C. A. W. Jeekel published a comprehensive listing of all known millipede genera and families described between 1758 and 1957 in his Nomenclator Generum et Familiarum Diplopodorum, a work credited as launching the "modern era" of millipede taxonomy.[16][17] In 1980, the American biologist Richard L. Hoffman published a classification of millipedes which recognized the Penicillata, Pentazonia, and Helminthomorpha,[18] and the first phylogenetic analysis of millipede orders using modern cladistic methods was published in 1984 by Henrik Enghoff of Denmark.[19] A 2003 classification by the American myriapodologist Rowland Shelley is similar to the one originally proposed by Verhoeff, and remains the currently accepted classification scheme (shown below), despite more recent molecular studies proposing conflicting relationships.[5][9] A 2011 summary of millipede family diversity by William A. Shear placed the order Siphoniulida within the larger group Nematophora.[3]
| Diplopoda |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fossil record
In addition to the 16 living orders, there are 9 extinct orders and one superfamily known only from fossils. The relationship of these to living groups and to each other is controversial. The extinct Arthropleuridea was long considered a distinct myriapod class, although work in the early 21st century established the group as a subclass of millipedes.[20][21][22] Several living orders also appear in the fossil record. Below are two proposed arrangements of fossil millipede groups.[5][9] Extinct groups are indicated with a dagger (†). The extinct order Zosterogrammida, a chilognath of uncertain position,[9] is not shown.
| |||||||||||||||||||||||||||
| Alternate hypothesis of fossil relationships[5][21] |
| Diplopoda |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Relation to other myriapods
Although the relationships of millipede orders are still the subject of debate, the class Diplopoda as a whole is considered a monophyletic group of arthropods: all millipedes are more closely related to each other than to any other arthropods. Diplopoda is a class within the arthropod subphylum Myriapoda, the myriapods, which includes centipedes (class Chilopoda) as well as the lesser-known pauropods (class Pauropoda) and symphylans (class Symphyla). Within myriapods, the closest relatives or sister group of millipedes has long been considered the pauropods, which also have a collum and diplosegments.[5]
Distinction from centipedes
The differences between millipedes and centipedes are a common question from the general public.[23] Both groups of myriapods share similarities, such as long, multi-segmented bodies, many legs, a single pair of antennae, and the presence of postanntennal organs, but have many differences and distinct evolutionary histories, as the most recent common ancestor of centipedes and millipedes lived around 450 to 475 million years ago in the Silurian.[24] The head alone exemplifies the differences; millipedes have short, elbowed antennae for probing the substrate, a pair of robust mandibles and a single pair of maxillae fused into a lip; centipedes have long, threadlike antennae, a pair of small mandibles, two pairs of maxillae and a pair of large poison claws.[25]
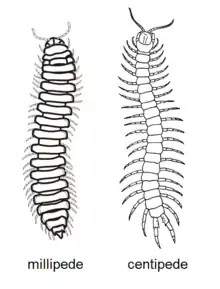
| Trait | Millipedes | Centipedes |
|---|---|---|
| Legs | Two pairs on most body segments; attached to underside of body | One pair per body segment; attached to sides of body; last pair extends backwards |
| Locomotion | Generally adapted for burrowing or inhabiting small crevices; slow-moving | Generally adapted for running, except for the burrowing soil centipedes |
| Feeding | Primarily detritivores, some herbivores, few carnivores; no venom | Primarily carnivores with claws modified into venomous fangs |
| Spiracles | On underside of body | On the sides or top of body |
| Reproductive openings | Third body segment | Last body segment |
| Reproductive behaviour | Male generally inserts spermatophore into female with gonopods | Male produces spermatophore that is usually picked up by female |
Characteristics

Millipedes come in a variety of body shapes and sizes, ranging from 2 mm (0.08 in) to around 35 cm (14 in) in length,[26] and can have as few as eleven to over a hundred segments.[27] They are generally black or brown in colour, although there are a few brightly coloured species, and some have aposematic colouring to warn that they are toxic.[2] Species of Motyxia produce cyanide as a chemical defence and are bioluminescent.[28]
Body styles vary greatly between major millipede groups. In the basal subclass Penicillata, consisting of the tiny bristle millipedes, the exoskeleton is soft and uncalcified, and is covered in prominent setae or bristles. All other millipedes, belonging to the subclass Chilognatha, have a hardened exoskeleton. The chilognaths are in turn divided into two infraclasses: the Pentazonia, containing relatively short-bodied groups such as pill millipedes, and the Helminthomorpha ("worm-like" millipedes), which contains the vast majority of species, with long, many-segmented bodies.[7][8]
Head
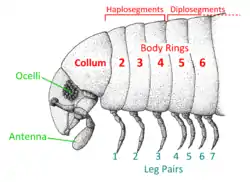
The head of a millipede is typically rounded above and flattened below and bears a pair of large mandibles in front of a plate-like structure called a gnathochilarium ("jaw lip").[5] The head contains a single pair of antennae with seven or eight segments and a group of sensory cones at the tip.[5] Many orders also possess a pair of sensory organs known as the Tömösváry organs, shaped as small oval rings posterior and lateral to the base of the antennae. Their function is unknown,[5] but they also occur in some centipedes, and are possibly used to measure humidity or light levels in the surrounding environment.[29]
Millipede eyes consist of several simple flat-lensed ocelli arranged in a group or patch on each side of the head. These patches are also called ocular fields or ocellaria. Many species of millipedes, including the entire orders Polydesmida, Siphoniulida, Glomeridesmida, Siphonophorida and Platydesmida, and cave-dwelling millipedes such as Causeyella and Trichopetalum, had ancestors that could see but have subsequently lost their eyes and are blind.[26]
Body

Millipede bodies may be flattened or cylindrical, and are composed of numerous metameric segments, each with an exoskeleton consisting of four chitinous plates: a single plate above (the tergite), one at each side (pleurites), and a plate on the underside (sternite) where the legs attach. In many millipedes, such as Merocheta and Juliformia, these plates are fused to varying degrees, sometimes forming a single cylindrical ring. The plates are typically hard, being impregnated with calcium salts.[27] Because they can't close their permanently open spiracles and most species lack a waxy cuticle, millipedes are susceptible to water loss and with a few exceptions must spend most of their time in moist or humid environments.[30]
The first segment behind the head is legless and known as a collum (from the Latin for neck or collar). The second, third, and fourth body segments bear a single pair of legs each and are known as "haplosegments" (the three haplosegments are sometimes referred to as a "thorax"[12]). The remaining segments, from the fifth to the posterior, are properly known as diplosegments or double segments, formed by the fusion of two embryonic segments. Each diplosegment bears two pairs of legs, rather than just one as in centipedes. In some millipedes, the last few segments may be legless. The terms "segment" or "body ring" are often used interchangeably to refer to both haplo- and diplosegments. The final segment is known as the telson and consists of a legless preanal ring, a pair of anal valves (closeable plates around the anus), and a small scale below the anus.[5][27]
Millipedes in several orders have keel-like extensions of the body-wall known as paranota, which can vary widely in shape, size, and texture; modifications include lobes, papillae, ridges, crests, spines and notches.[2] Paranota may allow millipedes to wedge more securely into crevices, protect the legs, or make the millipede more difficult for predators to swallow.[31]
The legs are composed of seven segments, and attach on the underside of the body. The legs of an individual are generally rather similar to each other, although often longer in males than females, and males of some species may have a reduced or enlarged first pair of legs.[32] The most conspicuous leg modifications are involved in reproduction, discussed below. Despite the common name, no millipede has been discovered with 1,000 legs: common species have between 34 and 400 legs, and the record is held by Illacme plenipes, with individuals possessing up to 750 legs – more than any other creature on Earth.[33]
_with_618_legs_-_ZooKeys-241-077-SP-6-top.jpg.webp)
Internal organs
Millipedes breathe through two pairs of spiracles located ventrally on each segment near the base of the legs.[23] Each opens into an internal pouch, and connects to a system of tracheae. The heart runs the entire length of the body, with an aorta stretching into the head. The excretory organs are two pairs of malpighian tubules, located near the mid-part of the gut. The digestive tract is a simple tube with two pairs of salivary glands to help digest the food.[27]
Reproduction and growth

Millipedes show a diversity of mating styles and structures. In the basal order Polyxenida (bristle millipedes), mating is indirect: males deposit spermatophores onto webs they secrete with special glands, and the spermatophores are subsequently picked up by females.[23] In all other millipede groups, males possess one or two pairs of modified legs called gonopods which are used to transfer sperm to the female during copulation. The location of the gonopods differs between groups: in males of the Pentazonia they are located at the rear of the body and known as telopods and may also function in grasping females, while in the Helminthomorpha – the vast majority of species – they are located on the seventh body segment.[5] A few species are parthenogenetic, having few, if any, males.[34]

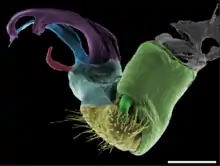
Gonopods occur in a diversity of shapes and sizes, and in the range from closely resembling walking legs to complex structures quite unlike legs at all. In some groups, the gonopods are kept retracted within the body; in others they project forward parallel to the body. Gonopod morphology is the predominant means of determining species among millipedes: the structures may differ greatly between closely related species but very little within a species.[35] The gonopods develop gradually from walking legs through successive moults until reproductive maturity.[36]
The genital openings (gonopores) of both sexes are located on the underside of the third body segment (near the second pair of legs) and may be accompanied in the male by one or two penes which deposit the sperm packets onto the gonopods. In the female, the genital pores open into paired small sacs called cyphopods or vulvae, which are covered by small hood-like lids, and are used to store the sperm after copulation.[27] The cyphopod morphology can also be used to identify species. Millipede sperm lack flagella, a unique trait among myriapods.[5]
In all except the bristle millipedes, copulation occurs with the two individuals facing one another. Copulation may be preceded by male behaviours such as tapping with antennae, running along the back of the female, offering edible glandular secretions, or in the case of some pill-millipedes, stridulation or "chirping".[37] During copulation in most millipedes, the male positions his seventh segment in front of the female's third segment, and may insert his gonopods to extrude the vulvae before bending his body to deposit sperm onto his gonopods and reinserting the "charged" gonopods into the female.[32]
Females lay from ten to three hundred eggs at a time, depending on species, fertilising them with the stored sperm as they do so. Many species deposit the eggs on moist soil or organic detritus, but some construct nests lined with dried faeces, and may protect the eggs within silk cocoons.[27] In most species, the female abandons the eggs after they are laid, but some species in the orders Platydesmida and Stemmiulida provide parental care for eggs and young.[23]
The young hatch after a few weeks, and typically have only three pairs of legs, followed by up to four legless segments. As they grow, they continually moult, adding further segments and legs as they do so. Some species moult within specially prepared chambers of soil or silk,[38] and may also shelter in these during wet weather, and most species eat the discarded exoskeleton after moulting. The adult stage, when individuals become reproductively mature, is generally reached in the final moult stage, which varies between species and orders, although some species continue to moult after adulthood. Furthermore, some species alternate between reproductive and non-reproductive stages after maturity, a phenomenon known as periodomorphosis, in which the reproductive structures regress during non-reproductive stages.[34] Millipedes may live from one to ten years, depending on species.[27]
Ecology
Habitat and distribution
Millipedes occur on all continents except Antarctica, and occupy almost all terrestrial habitats, ranging as far north as the Arctic Circle in Iceland, Norway, and Central Russia, and as far south as Santa Cruz Province, Argentina.[39][40] Typically forest floor dwellers, they live in leaf litter, dead wood, or soil, with a preference for humid conditions. In temperate zones, millipedes are most abundant in moist deciduous forests, and may reach densities of over 1,000 individuals per square metre. Other habitats include coniferous forests, caves, and alpine ecosystems.[23][40] Deserticolous millipedes, species evolved to live in the desert, like Orthoporus ornatus, may show adaptations like a waxy epicuticle and the ability of water uptake from unsaturated air.[41] Some species can survive freshwater floods and live submerged underwater for up to 11 months.[42][43] A few species occur near the seashore and can survive in somewhat salty conditions.[34][44]
Burrowing
The diplosegments of millipedes have evolved in conjunction with their burrowing habits, and nearly all millipedes adopt a mainly subterranean lifestyle. They use three main methods of burrowing; bulldozing, wedging and boring. Members of the orders Julida, Spirobolida and Spirostreptida, lower their heads and barge their way into the substrate, the collum being the portion of their exoskeleton that leads the way. Flat-backed millipedes in the order Polydesmida tend to insert their front end, like a wedge, into a horizontal crevice, and then widen the crack by pushing upwards with their legs, the paranota in this instance constituting the main lifting surface. Boring is used by members of the order Polyzoniida. These have smaller segments at the front and increasingly large ones further back; they propel themselves forward into a crack with their legs, the wedge-shaped body widening the gap as they go. Some millipedes have adopted an above-ground lifestyle and lost the burrowing habit. This may be because they are too small to have enough leverage to burrow, or because they are too large to make the effort worthwhile, or in some cases because they move relatively fast (for a millipede) and are active predators.[2]
Diet
Most millipedes are detritivores and feed on decomposing vegetation, feces, or organic matter mixed with soil. They often play important roles in the breakdown and decomposition of plant litter: estimates of consumption rates for individual species range from 1 to 11 percent of all leaf litter, depending on species and region, and collectively millipedes may consume nearly all the leaf litter in a region. The leaf litter is fragmented in the millipede gut and excreted as pellets of leaf fragments, algae, fungi, and bacteria, which facilitates decomposition by the microorganisms.[32] Where earthworm populations are low in tropical forests, millipedes play an important role in facilitating microbial decomposition of the leaf litter.[2] Some millipedes are herbivorous, feeding on living plants, and some species can become serious pests of crops. Millipedes in the order Polyxenida graze algae from bark, and Platydesmida feed on fungi.[5] A few species are omnivorous or in Callipodida and Chordeumatida occasionally carnivorous,[45] feeding on insects, centipedes, earthworms, or snails.[27][46] Some species have piercing mouth parts that allow them to suck up plant juices.[23]
Predators and parasites

Millipedes are preyed on by a wide range of animals, including various reptiles, amphibians, birds, mammals, and insects.[5] Mammalian predators such as coatis and meerkats roll captured millipedes on the ground to deplete and rub off their defensive secretions before consuming their prey,[47] and certain poison dart frogs are believed to incorporate the toxic compounds of millipedes into their own defences.[48] Several invertebrates have specialised behaviours or structures to feed on millipedes, including larval glowworm beetles,[49] Probolomyrmex ants,[50] chlamydephorid slugs,[51] and predaceous dung beetles of the genera Sceliages and Deltochilum.[52][53] A large subfamily of assassin bugs, the Ectrichodiinae with over 600 species, has specialized in preying upon millipedes.[54] Parasites of millipedes include nematodes, phaeomyiid flies, and acanthocephalans.[5] Nearly 30 fungal species of the order Laboulbeniales have been found growing externally on millipedes, but some species may be commensal rather than parasitic.[55]
Defence mechanisms

Due to their lack of speed and their inability to bite or sting, millipedes' primary defence mechanism is to curl into a tight coil – protecting their delicate legs inside an armoured exoskeleton.[56]
Many species also emit various foul-smelling liquid secretions through microscopic holes called ozopores (the openings of "odoriferous" or "repugnatorial glands"), along the sides of their bodies as a secondary defence. Among the many irritant and toxic chemicals found in these secretions are alkaloids, benzoquinones, phenols, terpenoids, and hydrogen cyanide.[57][58] Some of these substances are caustic and can burn the exoskeleton of ants and other insect predators, and the skin and eyes of larger predators. Primates such as capuchin monkeys and lemurs have been observed intentionally irritating millipedes in order to rub the chemicals on themselves to repel mosquitoes.[59][60][61] Some of these defensive compounds also show antifungal activity.[62]
The bristly millipedes (order Polyxenida) lack both an armoured exoskeleton and odiferous glands, and instead are covered in numerous bristles that in at least one species, Polyxenus fasciculatus, detach and entangle ants.[63]
Other inter-species interactions

Some millipedes form mutualistic relationships with organisms of other species, in which both species benefit from the interaction, or commensal relationships, in which only one species benefits while the other is unaffected. Several species form close relationships with ants, a relationship known as myrmecophily, especially within the family Pyrgodesmidae (Polydesmida), which contains "obligate myrmecophiles", species which have only been found in ant colonies. More species are "facultative myrmecophiles", being non-exclusively associated with ants, including many species of Polyxenida that have been found in ant nests around the world.[64]
Many millipede species have commensal relationships with mites of the orders Mesostigmata and Astigmata. Many of these mites are believed to be phoretic rather than parasitic, which means that they use the millipede host as a means of dispersal.[65][66]
A novel interaction between millipedes and mosses was described in 2011, in which individuals of the newly discovered Psammodesmus bryophorus was found to have up to ten species living on its dorsal surface, in what may provide camouflage for the millipede and increased dispersal for the mosses.[67][68]
Interactions with humans
_Mantadia.jpg.webp)
Millipedes generally have little impact on human economic or social well-being, especially in comparison with insects, although locally they can be a nuisance or agricultural pest. Millipedes do not bite, and their defensive secretions are mostly harmless to humans — usually causing only minor discolouration on the skin — but the secretions of some tropical species may cause pain, itching, local erythema, edema, blisters, eczema, and occasionally cracked skin.[69][70][71][72] Eye exposures to these secretions causes general irritation and potentially more severe effects such as conjunctivitis and keratitis.[73] This is called millipede burn. First aid consists of flushing the area thoroughly with water; further treatment is aimed at relieving the local effects.

Some millipedes are considered household pests, including Xenobolus carnifex which can infest thatched roofs in India,[74] and Ommatoiulus moreleti, which periodically invades homes in Australia. Other species exhibit periodical swarming behaviour, which can result in home invasions,[75] crop damage,[76] and train delays when the tracks become slippery with the crushed remains of hundreds of millipedes.[32][77][78] Some millipedes can cause significant damage to crops: the spotted snake millipede (Blaniulus guttulatus) is a noted pest of sugar beets and other root crops, and as a result is one of the few millipedes with a common name.[34]
Some of the larger millipedes in the orders Spirobolida, Spirostreptida, and Sphaerotheriida are popular as pets.[79] Some species commonly sold or kept include species of Archispirostreptus, Aphistogoniulus, Narceus, and Orthoporus.[80]

Millipedes appear in folklore and traditional medicine around the world. Some cultures associate millipede activity with coming rains.[81] In Zambia, smashed millipede pulp is used to treat wounds, and the Bafia people of Cameroon use millipede juice to treat earache.[81] In certain Himalayan Bhotiya tribes, dry millipede smoke is used to treat haemorrhoids.[82] Native people in Malaysia use millipede secretions in poison-tipped arrows.[81] The secretions of Spirobolus bungii have been observed to inhibit division of human cancer cells.[83] The only recorded usage of millipedes as food by humans comes from the Bobo people of Burkina Faso in West Africa, who consume boiled, dried millipedes belonging to the families Gomphodesmidae and Spirostreptidae in tomato sauce.[84]
Millipedes have also inspired and played roles in scientific research. In 1963, a walking vehicle with 36 legs was designed, said to have been inspired by a study of millipede locomotion.[85] Experimental robots have had the same inspiration,[86][87] in particular when heavy loads are needed to be carried in tight areas involving turns and curves.[88] In biology, some authors have advocated millipedes as model organisms for the study of arthropod physiology and the developmental processes controlling the number and shape of body segments.[32]
References
- Hoffman, Richard L. (1990). "Diplopoda". In Dindal, Daniel L. (ed.). Soil Biology Guide. John Wiley & Sons. p. 835. ISBN 978-0-471-04551-9.
Hoffman, Richard L. (2000). "Milliped or Millipede?" (PDF). Bulletin of the British Myriapod Group. 16: 36–37. - Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 711–717. ISBN 978-81-315-0104-7.
- Shear, W. (2011). "Class Diplopoda de Blainville in Gervais, 1844. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness" (PDF). Zootaxa. 3148: 159–164. doi:10.11646/zootaxa.3148.1.32.
- Brewer, Michael S.; Sierwald, Petra; Bond, Jason E. (2012). "Millipede taxonomy after 250 years: Classification and taxonomic practices in a mega-diverse yet understudied arthropod group". PLOS ONE. 7 (5): e37240. Bibcode:2012PLoSO...737240B. doi:10.1371/journal.pone.0037240. PMC 3352885. PMID 22615951.
- Sierwald, Petra; Bond, Jason E. (2007). "Current status of the myriapod class Diplopoda (Millipedes): Taxonomic diversity and phylogeny". Annual Review of Entomology. 52 (1): 401–420. doi:10.1146/annurev.ento.52.111805.090210. PMID 17163800.
- Barker, G.M. (2004). Natural Enemies of Terrestrial Molluscs. CABI. pp. 405–406. ISBN 978-0-85199-061-3.
- Bueno-Villegas, Julián; Sierwald, Petra; Bond, Jason E. "Diplopoda" (PDF). In Bousquets, J. L.; Morrone, J. J. (eds.). Biodiversidad, taxonomia y biogeografia de artropodos de Mexico. pp. 569–599.
- Shelley, Rowland M. "Millipedes". University of Tennessee: Entomology and Plant Pathology. Retrieved 17 July 2016.
- Shear, William A.; Edgecombe, Gregory D. (2010). "The geological record and phylogeny of the Myriapoda". Arthropod Structure & Development. 39 (2–3): 174–190. doi:10.1016/j.asd.2009.11.002. PMID 19944188.
- Hoffman, R. L. (1963). "New genera and species of Upper Paleozoic Diplopoda". Journal of Paleontology. 37 (1): 167–174. JSTOR 1301419.
- Garwood, Russell; Edgecombe, Gregory (2011). "Early terrestrial animals, evolution and uncertainty". Evolution: Education and Outreach. 4 (3): 489–501. doi:10.1007/s12052-011-0357-y.
- Wilson, Heather M.; Anderson, Lyall I. (2004). "Morphology and taxonomy of Paleozoic millipedes (Diplopoda: Chilognatha: Archipolypoda) from Scotland". Journal of Paleontology. 78 (1): 169–184. doi:10.1666/0022-3360(2004)078<0169:MATOPM>2.0.CO;2.
- Sues, Hans-Dieter (15 January 2011). "Largest Land-Dwelling "Bug" of All Time". National Geographic. Archived from the original on 4 March 2016. Retrieved 25 February 2016.
- Lockley, M. G.; Meyer, Christian (2013). "The Tradition of Tracking Dinosaurs in Europe". Dinosaur Tracks and Other Fossil Footprints of Europe. Columbia University Press. pp. 25–52. ISBN 978-0-231-50460-7.
- Caroli Linnaei (1758). Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. v.1. Impensis Direct. Laurentii Salvii. pp. 639–640.
- Shelley, R. M. (2007). "Taxonomy of extant Diplopoda (Millipeds) in the modern era: Perspectives for future advancements and observations on the global diplopod community (Arthropoda: Diplopoda)" (PDF). Zootaxa. 1668: 343–362. doi:10.11646/zootaxa.1668.1.18.
- Shelley, Rowland M.; Sierwald, Petra; Kiser, Selena B.; Golovatch, Sergei I. (2000). Nomenclator generum et familiarum Diplopodorum II : a list of the genus and family-group names in the class Diplopoda from 1958 through 1999. Sofia, Bulgaria: Pensoft. p. 5. ISBN 978-954-642-107-4.
- Hoffman, Richard L. (1980). Classification of the Diplopoda. Geneva, Switzerland: Muséum d'Historie Naturelle. pp. 1–237.
- Enghoff, H. (1984). "Phylogeny of millipedes – a cladistic analysis". Journal of Zoological Systematics and Evolutionary Research. 22 (1): 8–26. doi:10.1111/j.1439-0469.1984.tb00559.x.
- Wilson, Heather M.; Shear, William A. (2000). "Microdecemplicida, a new order of minute arthropleurideans (Arthropoda: Myriapoda) from the Devonian of New York State, U.S.A". Transactions of the Royal Society of Edinburgh: Earth Sciences. 90 (4): 351–375. doi:10.1017/S0263593300002674.
- Kraus, O.; Brauckmann, C. (2003). "Fossil giants and surviving dwarfs. Arthropleurida and Pselaphognatha (Atelocerata, Diplopoda): characters, phylogenetic relationships and construction". Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg. 40: 5–50.
- Kraus, O. (2005). "On the structure and biology of Arthropleura species (Atelocerata, Diplopoda; Upper Carboniferous/Lower Permian)". Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg. 41: 5–23.
- Shelley, Rowland M. (1999). "Centipedes and millipedes with emphasis on North American fauna". The Kansas School Naturalist. 45 (3): 1–16. Archived from the original on 2016-11-12. Retrieved 2013-10-14.
- Brewer, Michael S.; Bond, Jason E. (2013). "Ordinal-level phylogenomics of the arthropod class Diplopoda (Millipedes) based on an analysis of 221 nuclear protein-coding loci generated using next-generation sequence analyses". PLOS ONE. 8 (11): e79935. Bibcode:2013PLoSO...879935B. doi:10.1371/journal.pone.0079935. PMC 3827447. PMID 24236165.
- Blower, John Gordon (1985). Millipedes: Keys and Notes for the Identification of the Species. Brill Archive. p. 1. ISBN 978-90-04-07698-3.
- Minelli, Alessandro; Golovatch, Sergei I. (2001). "Myriapods" (PDF). In Levin, Simon A. (ed.). Encyclopedia of Biodiversity. pp. 291–303. ISBN 978-0-12-226865-6. Archived from the original (PDF) on 2014-02-21.
- Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 818–825. ISBN 978-0-03-056747-6.
- Marek, Paul E.; Moore, Wendy (2015). "Discovery of a glowing millipede in California and the gradual evolution of bioluminescence in Diplopoda". Proceedings of the National Academy of Sciences. 112 (20): 6419–6424. Bibcode:2015PNAS..112.6419M. doi:10.1073/pnas.1500014112. PMC 4443369. PMID 25941389.
- Lewis, J. G. E. (2008). The Biology of Centipedes (Digitally printed 1st paperback version. ed.). Cambridge: Cambridge University Press. pp. 110–111. ISBN 978-0-521-03411-1.
- Capinera, John L., ed. (2008). "Millipedes". Encyclopedia of Entomology. Springer. pp. 2395–2397. ISBN 978-1-4020-6242-1.
- Mesibov, Robert. "Paranota". External Anatomy of Polydesmida. Archived from the original on 4 March 2016. Retrieved 30 October 2013.
- Hopkin, Stephen P.; Read, Helen J. (1992). The Biology of Millipedes. Oxford University Press. ISBN 978-0-19-857699-0.
- Marek, P.; Shear, W.; Bond, J. (2012). "A redescription of the leggiest animal, the millipede Illacme plenipes, with notes on its natural history and biogeography (Diplopoda, Siphonophorida, Siphonorhinidae)". ZooKeys (241): 77–112. doi:10.3897/zookeys.241.3831. PMC 3559107. PMID 23372415.
- Blower, J. Gordon (1985). Millipedes: Keys and Notes for the Identification of the Species. London: Published for the Linnean Society of London and the Estuarine and Brackish-Water Sciences Association by E.J. Brill. ISBN 978-90-04-07698-3.
- Mesibov, Robert. "Gonopods". External Anatomy of Polydesmida. Archived from the original on 9 July 2017. Retrieved 27 October 2013.
- Drago, Leandro; Fusco, Giuseppe; Garollo, Elena; Minelli, Alessandro (2011). "Structural aspects of leg-to-gonopod metamorphosis in male helminthomorph millipedes (Diplopoda)". Frontiers in Zoology. 8 (1): 19. doi:10.1186/1742-9994-8-19. PMC 3170261. PMID 21859471.
- Wesener, Thomas; Köhler, Jörn; Fuchs, Stefan; van den Spiegel, Didier (2011). "How to uncoil your partner—"mating songs" in giant pill-millipedes (Diplopoda: Sphaerotheriida)". Naturwissenschaften. 98 (11): 967–975. Bibcode:2011NW.....98..967W. doi:10.1007/s00114-011-0850-8. PMID 21971844. S2CID 12005617.
- Enghoff, Henrik; Akkari, Nesrine (2011). "A callipodidan cocoon (Diplopoda, Callipodida, Schizopetalidae)". International Journal of Myriapodology. 5: 49–53. doi:10.3897/ijm.5.1995.
- Shelley, Rowland M.; Golavatch, Sergei I. (2011). "Atlas of myriapod biogeography. I. Indigenous ordinal and supra-ordinal distributions in the Diplopoda: Perspectives on taxon origins and ages, and a hypothesis on the origin and early evolution of the class". Insecta Mundi. 158: 1–134.
- Golovatch, Sergei I.; Kime, R. Desmond (2009). "Millipede (Diplopoda) distributions: a review" (PDF). Soil Organisms. 81 (3): 565–597. Archived from the original (PDF) on 2016-03-03. Retrieved 2014-11-19.
- Millipede (Diplopoda) distributions: A review
- Adis, Joachim (1986). "An 'aquatic' millipede from a Central Amazonian inundation forest". Oecologia. 68 (3): 347–349. Bibcode:1986Oecol..68..347A. doi:10.1007/BF01036737. PMID 28311777. S2CID 11374324.
- Burrows, F. J.; Hales, D. F.; Beattie, A. J. (1994). "Aquatic millipedes in Australia: a biological enigma and a conservation saga". Australian Zoologist. 29 (3–4): 213–216. doi:10.7882/az.1994.007.
- Barber, A. D., ed. (2013). "World Database of Littoral Myriapoda". World Register of Marine Species. Retrieved 25 October 2013.
- Treatise on Zoology - Anatomy, Taxonomy, Biology. The Myriapoda, Volume 2
- Barker, G. M. (2004). "Millipedes (Diplopoda) and Centipedes (Chilopoda) (Myriapoda) as predators of terrestrial gastropods". In Barker, G. M. (ed.). Natural Enemies of Terrestrial Molluscs. CAB International. pp. 405–426. ISBN 978-0-85199-061-3.
- Weldon, Paul J.; Cranmore, Catherine F.; Chatfield, Jenifer A. (2006). "Prey-rolling behavior of coatis (Nasua spp.) is elicited by benzoquinones from millipedes". Naturwissenschaften. 93 (1): 14–16. Bibcode:2006NW.....93...14W. doi:10.1007/s00114-005-0064-z. PMID 16391932. S2CID 22200949.
- Saporito, R. A.; Donnelly, M. A.; Hoffman, R. L.; Garraffo, H. M.; Daly, J. W. (2003). "A siphonotid millipede (Rhinotus) as the source of spiropyrrolizidine oximes of dendrobatid frogs". Journal of Chemical Ecology. 29 (12): 2781–2786. doi:10.1023/B:JOEC.0000008065.28364.a0. PMID 14969363. S2CID 4094895.
- Eisner, T.; Eisner, M.; Attygalle, A. B.; Deyrup, M.; Meinwald, J. (1998). "Rendering the inedible edible: circumvention of a millipede's chemical defence by a predaceous beetle larva". Proceedings of the National Academy of Sciences of the United States of America. 95 (3): 1108–13. Bibcode:1998PNAS...95.1108E. doi:10.1073/pnas.95.3.1108. PMC 18689. PMID 9448293.
- Ito, F. (1998). "Colony composition and specialized predation on millipedes in the enigmatic ponerine ant genus Probolomyrmex (Hymenoptera, Formicidae)" (PDF). Insectes Sociaux. 45 (1): 79–83. doi:10.1007/s000400050070. S2CID 22119946.
- Herbert, D. G. (2000). "Dining on diplopods: remarkable feeding behaviour in chlamydephorid slugs (Mollusca: Gastropoda)". Journal of Zoology. 251 (1): 1–5. doi:10.1111/j.1469-7998.2000.tb00586.x.
- Forgie, Shaun A.; Grebennikov, Vasily V.; Scholtz, Clarke H. (2002). "Revision of Sceliages Westwood, a millipede-eating genus of southern African dung beetles (Coleoptera : Scarabaeidae)". Invertebrate Systematics. 16 (6): 931–955. doi:10.1071/IT01025.
- Larsen, T. H; Lopera, A.; Forsyth, A.; Genier, F. (2009). "From coprophagy to predation: a dung beetle that kills millipedes". Biology Letters. 5 (2): 152–155. doi:10.1098/rsbl.2008.0654. PMC 2665820. PMID 19158030.
- Forthman, M.; Weirauch, C. (2012). "Toxic associations: a review of the predatory behaviors of millipede assassin bugs (Hemiptera: Reduviidae: Ectrichodiinae)" (PDF). European Journal of Entomology. 109 (2): 147–153. doi:10.14411/eje.2012.019.
- Santamaría, Sergi; Enghoff, Henrik; Reboleira, Ana Sofía P.S. (2018). "New species of Troglomyces and Diplopodomyces (Laboulbeniales, Ascomycota) from millipedes (Diplopoda)". European Journal of Taxonomy (429). doi:10.5852/ejt.2018.429.
- Animals: The International Wildlife Magazine. Nigel-Sitwell. 1964. p. 21.
- Blum, Murray S.; Woodring, J. Porter (1962). "Secretion of benzaldehyde and hydrogen cyanide by the millipede Pachydesmus crassicutis (Wood)". Science. 138 (3539): 512–513. Bibcode:1962Sci...138..512B. doi:10.1126/science.138.3539.512. PMID 17753947. S2CID 40193390.
- Kuwahara, Yasumasa; Ômura, Hisashi; Tanabe, Tsutomu (2002). "2-Nitroethenylbenzenes as natural products in millipede defense secretions". Naturwissenschaften. 89 (7): 308–310. Bibcode:2002NW.....89..308K. doi:10.1007/s00114-002-0328-9. PMID 12216861. S2CID 30068731.
- Weldon, Paul J.; Aldich, Jeffrey R.; Klun, Jerome A.; Oliver, James E.; Debboun, Mustapha (2003). "Benzoquinones from millipedes deter mosquitoes and elicit self-anointing in capuchin monkeys (Cebus spp.)". Naturwissenschaften. 90 (7): 301–305. Bibcode:2003NW.....90..301W. doi:10.1007/s00114-003-0427-2. PMID 12883771. S2CID 15161505. Retrieved 2018-04-29.
- Valderrama, Ximena; Robinson, John G.; Attygalle, Athula B.; Eisner, Thomas (2000). "Seasonal anointment with millipedes in a wild primate: a chemical defense against insects". Journal of Chemical Ecology. 26 (12): 2781–2790. doi:10.1023/A:1026489826714. S2CID 25147071.
- Birkinshaw, Christopher R. (1999). "Use of millipedes by black lemurs to anoint their bodies". Folia Primatologica. 70 (3): 170–171. doi:10.1159/000021691. PMID 10394067. S2CID 36036598.
- Roncadori, R. W.; Duffey, S. S.; Blum, M. S. (1985). "Antifungal activity of defensive secretions of certain millipedes". Mycologia. 77 (2): 185–191. doi:10.2307/3793067. JSTOR 3793067.
- Eisner, Thomas; Eisner, Maria; Deyrup, Mark (1996). "Millipede defense: use of detachable bristles to entangle ants". Proceedings of the National Academy of Sciences. 93 (20): 10848–10851. Bibcode:1996PNAS...9310848E. doi:10.1073/pnas.93.20.10848. PMC 38244. PMID 8855269.
- Stoev, Pavel; Lapeva-Gjonova, Albena (2005). "Myriapods from ant nests in Bulgaria (Chilopoda, Diplopoda)" (PDF). Peckiana. 4: 131–142. Archived from the original (PDF) on 2016-03-03. Retrieved 2014-06-05.
- Farfan, Monica; Klompen, Hans (2012). "Phoretic mite associates of millipedes (Diplopoda, Julidae) in the northern Atlantic region (North America, Europe)". International Journal of Myriapodology. 7: 69–91. doi:10.3897/ijm.7.3064.
- Swafford, Lynn; Bond, Jason E. (2010). "Failure to cospeciate: an unsorted tale of millipedes and mites". Biological Journal of the Linnean Society. 101 (2): 272–287. doi:10.1111/j.1095-8312.2010.01499.x.
- Martínez-Torres, Shirley Daniella; Daza, Álvaro Eduardo Flórez; Linares-Castillo, Edgar Leonardo (2011). "Meeting between kingdoms: discovery of a close association between Diplopoda and Bryophyta in a transitional Andean-Pacific forest in Colombia". International Journal of Myriapodology. 6: 29–36. doi:10.3897/ijm.6.2187.
- Marshall, Michael (22 September 2011). "Zoologger: Stealth millipede wears living camouflage". New Scientist. Retrieved 26 June 2016.
- Mason, G.; Thompson, H.; Fergin, P.; Anderson, R. (1994). "Spot diagnosis: the burning millipede". Medical Journal of Australia. 160 (11): 718–726. doi:10.5694/j.1326-5377.1994.tb125915.x. PMID 8202008. S2CID 204065414.
- Shpall, S.; Frieden, I. (1991). "Mahogany discoloration of the skin due to the defensive secretion of a millipede". Pediatric Dermatology. 8 (1): 25–27. doi:10.1111/j.1525-1470.1991.tb00834.x. PMID 1862020. S2CID 1725209.
- Radford, A. (1976). "Giant millipede burns in Papua New Guinea". Papua New Guinea Medical Journal. 18 (3): 138–141. PMID 1065155.
- Radford, A. (1975). "Millipede burns in man". Tropical and Geographical Medicine. 27 (3): 279–287. PMID 1103388.
- Hudson, B.; Parsons, G. (1997). "Giant millipede 'burns' and the eye". Transactions of the Royal Society of Tropical Medicine and Hygiene. 91 (2): 183–185. doi:10.1016/S0035-9203(97)90217-0. PMID 9196764.
- Alagesan, P.; Muthukrishnan, J. (2005). "Bioenergetics of the household pest, Xenobolus carnifex (Fabricius, 1775)" (PDF). Peckiana. 4: 3–14. Archived from the original (PDF) on 2016-03-03. Retrieved 2013-11-12.
- Enghoff, Henrik; Kebapći, Ümit (2008). "Calyptophyllum longiventre (Verhoeff, 1941) invading houses in Turkey, with the first description of the male (Diplopoda: Julida: Julidae)". Journal of Natural History. 42 (31–32): 2143–2150. doi:10.1080/00222930802196055. S2CID 84768307.
- Ebregt, E.; Struik, P. C.; Odongo, B.; Abidin, P. E. (2005). "Pest damage in sweet potato, groundnut and maize in north-eastern Uganda with special reference to damage by millipedes (Diplopoda)". NJAS – Wageningen Journal of Life Sciences. 53 (1): 49–69. doi:10.1016/S1573-5214(05)80010-7.
- Niijima, Keiko (2001). ヤケヤスデ列車を止める [A millipede outbreak (Oxidus gracilis, Koch) stopped trains]. Edaphologia (in Japanese) (68): 43–46. doi:10.20695/edaphologia.68.0_43. ISSN 0389-1445.
- Peckham, Matt (4 September 2013). "Millipedes – Yes, Millipedes – May Be Responsible for Australian Train Crash". Time Newsfeed. Time Magazine. Retrieved 31 October 2013.
- Stoev, Pavel; Zapparoli, Marzio; Golovatch, Sergei; Enghoff, Henrik; Akkari, Nesrine; Barber, Anthony (2010). "Myriapods (Myriapoda). Chapter 7.2. In: Roques et al. (Eds). Alien terrestrial arthropods of Europe". BIORISK – Biodiversity and Ecosystem Risk Assessment. 4: 97–130. doi:10.3897/biorisk.4.51.
- Lewbart, Gregory A., ed. (2011-09-20). Invertebrate Medicine (2nd ed.). Wiley-Blackwell. p. 255. ISBN 978-0-470-96078-3.
- Costa Neto, Eraldo M. (2007). "The perception of Diplopoda (Arthropoda, Myriapoda) by the inhabitants of the county of Pedra Branca, Santa Teresinha, Bahia, Brazil". Acta Biológica Colombiana. 12 (2): 123–134.
- Negi, C. S.; Palyal, V. S. (2007). "Traditional uses of animal and animal products in medicine and rituals by the Shoka tribes of district Pithoragarh, Uttaranchal, India" (PDF). Studies on Ethno-Medicine. 1 (1): 47–54. doi:10.1080/09735070.2007.11886300. S2CID 30993906.
- Jiang, T. L.; Feng, G. W.; Shen, J. H.; Li, L. F.; Fu, X. Q. (1981). "Observation of the effect of Spirobolus bungii extract on cancer cells". Journal of Traditional Chinese Medicine. 1 (1): 34–8. PMID 6926686.
- Enghoff, Henrik; Manno, Nicola; Tchibozo, Sévérin; List, Manuela; Schwarzinger, Bettina; Schoefberger, Wolfgang; Schwarzinger, Clemens; Paoletti, Maurizio G. (2014). "Millipedes as food for humans: their nutritional and possible antimalarial value: a first report". Evidence-Based Complementary and Alternative Medicine. 2014: 1–9. doi:10.1155/2014/651768. PMC 3945075. PMID 24688592.
- Information, Reed Business (25 April 1963). "Canada: Money in muskeg?". New Scientist: 198–199. ISSN 0262-4079.
- Avirovik, Dragan; Butenhoff, Bryan; Priya, Shashank (2014). "Millipede-inspired locomotion through novel U-shaped piezoelectric motors". Smart Materials and Structures. 23 (3): 037001. Bibcode:2014SMaS...23c7001A. doi:10.1088/0964-1726/23/3/037001.
- Wakimoto, Shuichi; Suzumori, Koichi; Kanda, Takefumi (2006). "A bio-mimetic amphibious soft cord robot". Nihon Kikai Gakkai Ronbunshu, C Hen/Transactions of the Japan Society of Mechanical Engineers, Part C (in Japanese and English). 72 (2): 471–477.
- Beattie, Andrew; Ehrlich, Paul (2001). Wild Solutions: How Biodiversity is Money in the Bank (2nd ed.). New Haven: Yale University Press. pp. 192–194. ISBN 978-0-300-10506-3.
External links
| Wikimedia Commons has media related to Diplopoda. |
| Wikispecies has information related to Diplopoda. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Millipede. |
- Milli-PEET: The Class Diplopoda – The Field Museum, Chicago
- Millipedes of Australia
- Diplopoda: Guide to New Zealand Soil Invertebrates – Massey University
- SysMyr, a myriapod taxonomy database
- British Myriapod & Isopod Group


