Bilateria
The bilateria /baɪləˈtɪəriə/ or bilaterians are animals with bilateral symmetry as an embryo, i.e. having a left and a right side that are mirror images of each other. This also means they have a head and a tail (anterior-posterior axis) as well as a belly and a back (ventral-dorsal axis).[2] Nearly all are bilaterally symmetrical as adults as well; the most notable exception is the echinoderms, which achieve secondary pentaradial symmetry as adults, but are bilaterally symmetrical during embryonic development.
| Bilaterians | |
|---|---|
 | |
| Diversity of bilaterians. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Subkingdom: | Eumetazoa |
| Clade: | ParaHoxozoa |
| Clade: | Bilateria Hatschek, 1888 |
| Phyla | |
| |
| Synonyms | |
|
Triploblasts Lankester, 1873 | |
Most animals are bilaterians, excluding sponges, ctenophores, placozoans and cnidarians. For the most part, bilateral embryos are triploblastic, having three germ layers: endoderm, mesoderm, and ectoderm. Except for a few phyla (i.e. flatworms and gnathostomulids), bilaterians have complete digestive tracts with a separate mouth and anus. Some bilaterians lack body cavities (acoelomates, i.e. Platyhelminthes, Gastrotricha and Gnathostomulida), while others display primary body cavities (deriving from the blastocoel, as pseudocoeloms) or secondary cavities (that appear de novo, for example the coelom).
Body plan
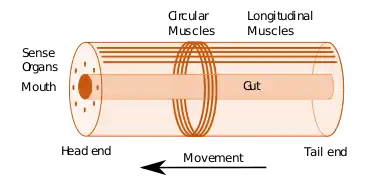
Some of the earliest bilaterians were wormlike, and a bilaterian body can be conceptualized as a cylinder with a gut running between two openings, the mouth and the anus. Around the gut it has an internal body cavity, a coelom or pseudocoelom.[lower-alpha 1] Animals with this bilaterally symmetric body plan have a head (anterior) end and a tail (posterior) end as well as a back (dorsal) and a belly (ventral); therefore they also have a left side and a right side.[4][2]
Having a front end means that this part of the body encounters stimuli, such as food, favouring cephalisation, the development of a head with sense organs and a mouth.[5] The body stretches back from the head, and many bilaterians have a combination of circular muscles that constrict the body, making it longer, and an opposing set of longitudinal muscles, that shorten the body;[2] these enable soft-bodied animals with a hydrostatic skeleton to move by peristalsis.[6] Most bilaterians (Nephrozoans) have a gut that extends through the body from mouth to anus, while Xenacoelomorphs have a bag gut with one opening. Many bilaterian phyla have primary larvae which swim with cilia and have an apical organ containing sensory cells. However, there are exceptions to each of these characteristics; for example, adult echinoderms are radially symmetric (unlike their larvae), and certain parasitic worms have extremely simplified body structures.[4][2]
Evolution

The hypothetical most recent common ancestor of all bilateria is termed the "Urbilaterian".[8][9] The nature of the first bilaterian is a matter of debate. One side suggests that acoelomates gave rise to the other groups (planuloid-aceloid hypothesis by Ludwig von Graff, Elie Metchnikoff, Libbie Hyman, or Luitfried von Salvini-Plawen), while the other poses that the first bilaterian was a coelomate organism and the main acoelomate phyla (flatworms and gastrotrichs) have lost body cavities secondarily (the Archicoelomata hypothesis and its variations such as the Gastrea by Haeckel or Sedgwick, the Bilaterosgastrea by Gösta Jägersten, or the Trochaea by Nielsen).
One hypothesis is that the original bilaterian was a bottom dwelling worm with a single body opening, similar to Xenoturbella.[3] It may have resembled the planula larvae of some cnidaria, which have some bilateral symmetry.[10]
Fossil record
The first evidence of bilateria in the fossil record comes from trace fossils in Ediacaran sediments, and the first bona fide bilaterian fossil is Kimberella, dating to 555 million years ago.[11] Earlier fossils are controversial; the fossil Vernanimalcula may be the earliest known bilaterian, but may also represent an infilled bubble.[12][13] Fossil embryos are known from around the time of Vernanimalcula (580 million years ago), but none of these have bilaterian affinities.[14] Burrows believed to have been created by bilaterian life forms have been found in the Tacuarí Formation of Uruguay, and are believed to be at least 585 million years old.[15]
Phylogeny
The Bilateria has traditionally been divided into two main lineages or superphyla.[16] The deuterostomes include the echinoderms, hemichordates, chordates, and a few smaller phyla. The protostomes include most of the rest, such as arthropods, annelids, mollusks, flatworms, and so forth. There are a number of differences, most notably in how the embryo develops. In particular, the first opening of the embryo becomes the mouth in protostomes, and the anus in deuterostomes. Many taxonomists now recognize at least two more superphyla among the protostomes, Ecdysozoa[17] (molting animals) and Spiralia.[17][18][19][20] The arrow worms (Chaetognatha) have proven difficult to classify; recent studies place them in the gnathifera.[21][22][23]
The traditional division of Bilateria into Deuterostomia and Protostomia was challenged when new morphological and molecular evidence found support for a sister relationship between the acoelomate taxa, Acoela and Nemertodermatida (together called Acoelomorpha), and the remaining bilaterians.[16] The latter clade was called Nephrozoa by Jondelius et al. (2002) and Eubilateria by Baguña and Riutort (2004).[16] The acoelomorph taxa had previously been considered flatworms with secondarily lost characteristics, but the new relationship suggested that the simple acoelomate worm form was the original bilaterian bodyplan and that the coelom, the digestive tract, excretory organs, and nerve cords developed in the Nephrozoa.[16][24] Subsequently the acoelomorphs were placed in phylum Xenacoelomorpha, together with the xenoturbellids, and the sister relationship between Xenacoelomorpha and Nephrozoa confirmed in phylogenomic analyses.[24]
A modern consensus phylogenetic tree for Bilateria is shown below, although the positions of certain clades are still controversial (dashed lines) and the tree has changed considerably since 2000.[25][23][26][27][28]
| Planulozoa |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 680 mya |
A different hypothesis is that the Ambulacraria are sister to Xenacoelomorpha together forming the Xenambulacraria. The Xenambulacraria may be sister to the Chordata or the Nephrozoa (sans Ambulacraria). The phylogenetic tree shown below depicts the latter proposal. Also the veracity of Deuterostomes is under discussion.[29][30][31][32] It is indicated when approximately clades radiated into newer clades in millions of years ago (Mya).[33] While the below tree depicts a chordates as a sister group to protostomia according to analyses by Philippe et al., the authors nonetheless caution that "the support values are very low, meaning there is no solid evidence to refute the traditional protostome and deuterostome dichotomy." [34]
| ParaHoxozoa |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 680 mya |
Notes
- The earliest Bilateria may have had only a single opening, and no coelom.[3]
References
- Martin, M. W.; Grazhdankin, D. V; Bowring, S. A; Evans, D. A; Fedonkin, M. A; Kirschvink, J. L. (5 May 2000). "Age of Neoproterozoic bilatarian [sic] body and trace fossils, White Sea, Russia: implications for metazoan evolution". Science. 288 (5467): 841–5. Bibcode:2000Sci...288..841M. doi:10.1126/science.288.5467.841. PMID 10797002. S2CID 1019572.
- Brusca, Richard C. (2016). "Introduction to the Bilateria and the Phylum Xenacoelomorpha: Triploblasty and Bilateral Symmetry Provide New Avenues for Animal Radiation" (PDF). Invertebrates. Sinauer Associates. pp. 345–372. ISBN 978-1-60535-375-3.
- Cannon, Johanna Taylor; Vellutini, Bruno Cossermelli; Smith, Julian; Ronquist, Fredrik; Jondelius, Ulf; Hejnol, Andreas (2016). "Xenacoelomorpha is the sister group to Nephrozoa". Nature. 530 (7588): 89–93. Bibcode:2016Natur.530...89C. doi:10.1038/nature16520. PMID 26842059. S2CID 205247296.
- Minelli, Alessandro (2009). Perspectives in Animal Phylogeny and Evolution. Oxford University Press. p. 53. ISBN 978-0-19-856620-5.
- Finnerty, John R. (November 2005). "Did internal transport, rather than directed locomotion, favor the evolution of bilateral symmetry in animals?" (PDF). BioEssays. 27 (11): 1174–1180. doi:10.1002/bies.20299. PMID 16237677. Archived from the original (PDF) on 2019-07-02. Retrieved 2018-03-07.
- Quillin, K. J. (May 1998). "Ontogenetic scaling of hydrostatic skeletons: geometric, static stress and dynamic stress scaling of the earthworm lumbricus terrestris". The Journal of Experimental Biology. 201 (12): 1871–83. PMID 9600869.
- Evans, Scott D.; Hughes, Ian V.; Gehling, James G.; Droser, Mary L. (7 April 2020). "Discovery of the oldest bilaterian from the Ediacaran of South Australia". Proceedings of the National Academy of Sciences. 117 (14): 7845–7850. doi:10.1073/pnas.2001045117. ISSN 0027-8424.
- Knoll, Andrew H.; Carroll, Sean B. (25 June 1999). "Early Animal Evolution: Emerging Views from Comparative Biology and Geology". Science. 284 (5423): 2129–2137. doi:10.1126/science.284.5423.2129. PMID 10381872. S2CID 8908451.
- Balavoine, G.; Adoutte, Andre (2003). "The segmented Urbilateria: A testable scenario". Integrative and Comparative Biology. 43 (1): 137–147. CiteSeerX 10.1.1.560.8727. doi:10.1093/icb/43.1.137. PMID 21680418. S2CID 80975506.
- Baguñà, Jaume; Martinez, Pere; Paps, Jordi; Riutort, Marta (April 2008). "Back in time: a new systematic proposal for the Bilateria". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1496): 1481–1491. doi:10.1098/rstb.2007.2238. PMC 2615819. PMID 18192186.
- Fedonkin, M. A.; Waggoner, B. M. (November 1997). "The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism". Nature. 388 (6645): 868–871. Bibcode:1997Natur.388..868F. doi:10.1038/42242. S2CID 4395089.
- Bengtson, S.; Budd, G. (19 November 2004). "Comment on 'small bilaterian fossils from 40 to 55 million years before the Cambrian'". Science. 306 (5700): 1291a. doi:10.1126/science.1101338. PMID 15550644.
- Bengtson, S.; Donoghue, P. C. J.; Cunningham, J. A.; Yin, C. (2012). "A merciful death for the 'earliest bilaterian,' Vernanimalcula". Evolution & Development. 14 (5): 421–427. doi:10.1111/j.1525-142X.2012.00562.x. PMID 22947315.
- Hagadorn, J. W.; Xiao, S.; Donoghue, P. C. J.; Bengtson, S.; Gostling, N. J.; Pawlowska, M.; Raff, E. C.; Raff, R. A.; Turner, F. R.; Chongyu, Y.; Zhou, C.; Yuan, X.; McFeely, M. B.; Stampanoni, M.; Nealson, K. H. (13 October 2006). "Cellular and Subcellular Structure of Neoproterozoic Animal Embryos". Science. 314 (5797): 291–294. Bibcode:2006Sci...314..291H. doi:10.1126/science.1133129. PMID 17038620. S2CID 25112751.
- Pecoits, E.; Konhauser, K. O.; Aubet, N. R.; Heaman, L. M.; Veroslavsky, G.; Stern, R. A.; Gingras, M. K. (June 29, 2012). "Bilaterian burrows and grazing behavior at >585 million years ago". Science. 336 (6089): 1693–1696. Bibcode:2012Sci...336.1693P. doi:10.1126/science.1216295. PMID 22745427. S2CID 27970523.
- Nielsen, Claus (2008). "Six major steps in animal evolution: are we derived sponge larvae?". Evol. Dev. 10 (2): 241–257. doi:10.1111/j.1525-142X.2008.00231.x. PMID 18315817.
- Halanych, K.; Bacheller, J.; Aguinaldo, A.; Liva, S.; Hillis, D.; Lake, J. (17 March 1995). "Evidence from 18S ribosomal DNA that the lophophorates are protostome animals". Science. 267 (5204): 1641–1643. Bibcode:1995Sci...267.1641H. doi:10.1126/science.7886451. PMID 7886451. S2CID 12196991.
- Paps, J.; Baguna, J.; Riutort, M. (14 July 2009). "Bilaterian phylogeny: a broad sampling of 13 nuclear genes provides a new Lophotrochozoa phylogeny and supports a paraphyletic basal Acoelomorpha". Molecular Biology and Evolution. 26 (10): 2397–2406. doi:10.1093/molbev/msp150. PMID 19602542.
- Telford, Maximilian J. (15 April 2008). "Resolving animal phylogeny: a sledgehammer for a tough nut?". Developmental Cell. 14 (4): 457–459. doi:10.1016/j.devcel.2008.03.016. PMID 18410719.
- "The Invertebrate Animals". Archived from the original on 2010-03-10. Retrieved 2006-01-09.
- Helfenbein, Kevin G.; Fourcade, H. Matthew; Vanjani, Rohit G.; Boore, Jeffrey L. (20 July 2004). "The mitochondrial genome of Paraspadella gotoi is highly reduced and reveals that chaetognaths are a sister group to protostomes". Proceedings of the National Academy of Sciences of the United States of America. 101 (29): 10639–10643. Bibcode:2004PNAS..10110639H. doi:10.1073/pnas.0400941101. PMC 489987. PMID 15249679.
- Papillon, Daniel; Perez, Yvan; Caubit, Xavier; Yannick Le, Parco (November 2004). "Identification of chaetognaths as protostomes is supported by the analysis of their mitochondrial genome". Molecular Biology and Evolution. 21 (11): 2122–2129. doi:10.1093/molbev/msh229. PMID 15306659.
- Fröbius, Andreas C.; Funch, Peter (2017-04-04). "Rotiferan Hox genes give new insights into the evolution of metazoan bodyplans". Nature Communications. 8 (1): 9. Bibcode:2017NatCo...8....9F. doi:10.1038/s41467-017-00020-w. PMC 5431905. PMID 28377584.
- Cannon, Johanna Taylor; Vellutini, Bruno Cossermelli; Smith, Julian; Ronquist, Fredrik; Jondelius, Ulf; Hejnol, Andreas (2016). "Xenacoelomorpha is the sister group to Nephrozoa". Nature. 530 (7588): 89–93. Bibcode:2016Natur.530...89C. doi:10.1038/nature16520. PMID 26842059. S2CID 205247296.
- Edgecombe, Gregory D.; Giribet, Gonzalo; Dunn, Casey W.; Hejnol, Andreas; Kristensen, Reinhardt M.; Neves, Ricardo C.; Rouse, Greg W.; Worsaae, Katrine; Sørensen, Martin V. (June 2011). "Higher-level metazoan relationships: recent progress and remaining questions". Organisms, Diversity & Evolution. 11 (2): 151–172. doi:10.1007/s13127-011-0044-4. S2CID 32169826.
- Smith, Martin R.; Ortega-Hernández, Javier (2014). "Hallucigenia's onychophoran-like claws and the case for Tactopoda" (PDF). Nature. 514 (7522): 363–366. Bibcode:2014Natur.514..363S. doi:10.1038/nature13576. PMID 25132546. S2CID 205239797.
- "Palaeos Metazoa: Ecdysozoa". palaeos.com. Retrieved 2017-09-02.
- Yamasaki, Hiroshi; Fujimoto, Shinta; Miyazaki, Katsumi (June 2015). "Phylogenetic position of Loricifera inferred from nearly complete 18S and 28S rRNA gene sequences". Zoological Letters. 1: 18. doi:10.1186/s40851-015-0017-0. PMC 4657359. PMID 26605063.
- Philippe, Hervé; Poustka, Albert J.; Chiodin, Marta; Hoff, Katharina J.; Dessimoz, Christophe; Tomiczek, Bartlomiej; Schiffer, Philipp H.; Müller, Steven; Domman, Daryl; Horn, Matthias; Kuhl, Heiner; Timmermann, Bernd; Satoh, Noriyuki; Hikosaka-Katayama, Tomoe; Nakano, Hiroaki; Rowe, Matthew L.; Elphick, Maurice R.; Thomas-Chollier, Morgane; Hankeln, Thomas; Mertes, Florian; Wallberg, Andreas; Rast, Jonathan P.; Copley, Richard R.; Martinez, Pedro; Telford, Maximilian J. (2019). "Mitigating Anticipated Effects of Systematic Errors Supports Sister-Group Relationship between Xenacoelomorpha and Ambulacraria". Current Biology. 29 (11): 1818–1826.e6. doi:10.1016/j.cub.2019.04.009. hdl:21.11116/0000-0004-DC4B-1. ISSN 0960-9822. PMID 31104936. S2CID 155104811.
- Philippe, Hervé; Brinkmann, Henner; Copley, Richard R.; Moroz, Leonid L.; Nakano, Hiroaki; Poustka, Albert J.; Wallberg, Andreas; Peterson, Kevin J.; Telford, Maximilian J. (2011). "Acoelomorph flatworms are deuterostomes related to Xenoturbella". Nature. 470 (7333): 255–258. Bibcode:2011Natur.470..255P. doi:10.1038/nature09676. ISSN 0028-0836. PMC 4025995. PMID 21307940.
- Rokhsar, Daniel S.; Satoh, Noriyuki; Goto, Taichiro; Peijnenburg, Katja T. C. A.; Marlétaz, Ferdinand (2019-01-21). "A New Spiralian Phylogeny Places the Enigmatic Arrow Worms among Gnathiferans". Current Biology. 29 (2): 312–318.e3. doi:10.1016/j.cub.2018.11.042. ISSN 0960-9822. PMID 30639106.
- Marlétaz, Ferdinand (2019-06-17). "Zoology: Worming into the Origin of Bilaterians". Current Biology. 29 (12): R577–R579. doi:10.1016/j.cub.2019.05.006. PMID 31211978.
- Peterson, Kevin J.; Cotton, James A.; Gehling, James G.; Pisani, Davide (2008-04-27). "The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records". Philosophical Transactions of the Royal Society of London B: Biological Sciences. 363 (1496): 1435–1443. doi:10.1098/rstb.2007.2233. PMC 2614224. PMID 18192191.
- Philippe, Hervé; Poustka, Albert J.; Chiodin, Marta; Hoff, Katharina J.; Dessimoz, Christophe; Tomiczek, Bartlomiej; Schiffer, Philipp H.; Müller, Steven; Domman, Daryl; Horn, Matthias; Kuhl, Heiner; Timmermann, Bernd; Satoh, Noriyuki; Hikosaka-Katayama, Tomoe; Nakano, Hiroaki; Rowe, Matthew L.; Elphick, Maurice R.; Thomas-Chollier, Morgane; Hankeln, Thomas; Mertes, Florian; Wallberg, Andreas; Rast, Jonathan P.; Copley, Richard R.; Martinez, Pedro; Telford, Maximilian J. (2019). "Mitigating Anticipated Effects of Systematic Errors Supports Sister-Group Relationship between Xenacoelomorpha and Ambulacraria". Current Biology. 29 (11): 1818–1826.e6. doi:10.1016/j.cub.2019.04.009. hdl:21.11116/0000-0004-DC4B-1. ISSN 0960-9822. PMID 31104936. S2CID 155104811.

.jpg.webp)
