Silver sulfate
Silver sulfate (Ag2SO4) is an ionic compound of silver used in silver plating and as a non-staining substitute to silver nitrate. This sulfate is stable under ordinary conditions of use and storage, though it darkens upon exposure to air or light. It is minimally soluble in water.
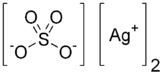 | |
 | |
| Names | |
|---|---|
| IUPAC name
Silver(I) sulfate | |
| Other names
Disilver sulfate Argentous sulfate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.581 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Ag2SO4 | |
| Molar mass | 311.79 g·mol−1 |
| Appearance | Colorless crystals |
| Odor | Odorless |
| Density | 5.45 g/cm3 (25 °C) 4.84 g/cm3 (660 °C)[1] |
| Melting point | 652.2–660 °C (1,206.0–1,220.0 °F; 925.4–933.1 K)[1][2] |
| Boiling point | 1,085 °C (1,985 °F; 1,358 K)[3][2] |
| 0.57 g/100 mL (0 °C) 0.69 g/100 mL (10 °C) 0.83 g/100 mL (25 °C) 0.96 g/100 mL (40 °C) 1.33 g/100 mL (100 °C)[4] | |
Solubility product (Ksp) |
1.2·10−5[1] |
| Solubility | Dissolves in aq. acids, alcohols, acetone, ether, acetates, amides[4] Insoluble in ethanol[3] |
| Solubility in sulfuric acid | 8.4498 g/L (0.1 molH2SO4/LH2O)[4] 25.44 g/100 g (13 °C) 31.56 g/100 g (24.5 °C) 127.01 g/100 g (96 °C)[3] |
| Solubility in ethanol | 7.109 g/L (0.5 nEtOH/H2O)[4] |
| Solubility in acetic acid | 7.857 g/L (0.5 nAcOH/H2O)[4] |
| −9.29·10−5 cm3/mol[1] | |
Refractive index (nD) |
nα = 1.756 nβ = 1.775 nγ = 1.782[5] |
| Structure | |
| Orthorhombic, oF56[5] | |
| Fddd, No. 70[5] | |
| 2/m 2/m 2/m[5] | |
α = 90°, β = 90°, γ = 90° | |
| Thermochemistry | |
Heat capacity (C) |
131.4 J/mol·K[1] |
Std molar entropy (S |
200.4 J/mol·K [1] |
Std enthalpy of formation (ΔfH⦵298) |
−715.9 kJ/mol[1] |
Gibbs free energy (ΔfG˚) |
−618.4 kJ/mol [1] |
| Hazards | |
| GHS pictograms |   [6] [6] |
| GHS Signal word | Danger |
| H318, H410[6] | |
| P273, P280, P305+351+338, P501[6] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and structure
Silver sulfate is prepared by adding sulfuric acid to a solution of silver nitrate:
- AgNO3 + H2SO4 → AgHSO4 + HNO3
- 2 AgHSO4 ⇌ Ag2SO4 + H2SO4
The compound adopts the structure seen for anhydrous sodium sulfate.[7]
Silver(II) sulfate
The synthesis of silver(II) sulfate (AgSO4) with a divalent silver ion instead of a monovalent silver ion was first reported in 2010[8] by adding sulfuric acid to silver(II) fluoride (HF escapes). It is a black solid that decomposes exothermally at 120 °C with evolution of oxygen and the formation of the pyrosulfate.
References
- Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- "MSDS of Silver sulfate". Fisher Scientific, Inc. Retrieved 2014-07-19.
- Anatolievich, Kiper Ruslan. "silver sulfate". Retrieved 2014-07-19.
- Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. pp. 622–623.
- Morris, Marlene C.; McMurdie, Howard F.; Evans, Eloise H.; Paretzkin, Boris; Groot, Johan H. de; Hubbard, Camden R.; Carmel, Simon J. (June 1976). "13". Standard X-ray Diffraction Powder Patterns. 25. Washington: Institute for Materials Research National Bureau of Standards.
- Sigma-Aldrich Co., Silver sulfate. Retrieved on 2014-07-19.
- Zachariasen, W. H. (1932). "Note on the Crystal Structure of Silver Sulphate, Ag2SO4". Zeitschrift für Kristallographie - Crystalline Materials. 82 (1–6). doi:10.1524/zkri.1932.82.1.161.
- Malinowski, P.; Derzsi, M.; Mazej, Z.; Jagličić, Z.; Gaweł, B.; Lasocha, W.; Grochala, W. (2010). "Ag(II)SO(4): A Genuine Sulfate of Divalent Silver with Anomalously Strong One-Dimensional Antiferromagnetic Interactions". Angewandte Chemie International Edition in English. 49 (9): 1683–1686. doi:10.1002/anie.200906863. PMID 20084660.
