Niobium
Niobium, also known as columbium, is a chemical element with the symbol Nb (formerly Cb) and atomic number 41. Niobium is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to that of pure titanium,[2] and it has similar ductility to iron. Niobium oxidizes in the earth's atmosphere very slowly, hence its application in jewelry as a hypoallergenic alternative to nickel. Niobium is often found in the minerals pyrochlore and columbite, hence the former name "columbium". Its name comes from Greek mythology, specifically Niobe, who was the daughter of Tantalus, the namesake of tantalum. The name reflects the great similarity between the two elements in their physical and chemical properties, making them difficult to distinguish.[3]
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Niobium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /naɪˈoʊbiəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | gray metallic, bluish when oxidized | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Nb) | 92.90637(1)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Niobium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d4 5s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 12, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2750 K (2477 °C, 4491 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 5017 K (4744 °C, 8571 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.57 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 30 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 689.9 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.60 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −3, −1, 0, +1, +2, +3, +4, +5 (a mildly acidic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 146 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 164±6 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Color lines in a spectral range | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc)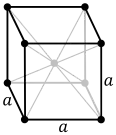 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 3480 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 7.3 µm/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 53.7 W/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 152 nΩ·m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 105 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 38 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 870–1320 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 735–2450 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-03-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Niobe in Greek mythology, daughter of Tantalus (tantalum) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Charles Hatchett (1801) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Christian Wilhelm Blomstrand (1864) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recognized as a distinct element by | Heinrich Rose (1844) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of niobium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The English chemist Charles Hatchett reported a new element similar to tantalum in 1801 and named it columbium. In 1809, the English chemist William Hyde Wollaston wrongly concluded that tantalum and columbium were identical. The German chemist Heinrich Rose determined in 1846 that tantalum ores contain a second element, which he named niobium. In 1864 and 1865, a series of scientific findings clarified that niobium and columbium were the same element (as distinguished from tantalum), and for a century both names were used interchangeably. Niobium was officially adopted as the name of the element in 1949, but the name columbium remains in current use in metallurgy in the United States.
It was not until the early 20th century that niobium was first used commercially. Brazil is the leading producer of niobium and ferroniobium, an alloy of 60–70% niobium with iron. Niobium is used mostly in alloys, the largest part in special steel such as that used in gas pipelines. Although these alloys contain a maximum of 0.1%, the small percentage of niobium enhances the strength of the steel. The temperature stability of niobium-containing superalloys is important for its use in jet and rocket engines.
Niobium is used in various superconducting materials. These superconducting alloys, also containing titanium and tin, are widely used in the superconducting magnets of MRI scanners. Other applications of niobium include welding, nuclear industries, electronics, optics, numismatics, and jewelry. In the last two applications, the low toxicity and iridescence produced by anodization are highly desired properties. Niobium is considered a technology-critical element.
History

_-_n._2990_-_Niobe_madre_-_Firenze.jpg.webp)
Niobium was identified by English chemist Charles Hatchett in 1801.[4][5][6] He found a new element in a mineral sample that had been sent to England from Connecticut, United States in 1734 by John Winthrop F.R.S. (grandson of John Winthrop the Younger) and named the mineral columbite and the new element columbium after Columbia, the poetical name for the United States.[7][8][9] The columbium discovered by Hatchett was probably a mixture of the new element with tantalum.[7]
Subsequently, there was considerable confusion[10] over the difference between columbium (niobium) and the closely related tantalum. In 1809, English chemist William Hyde Wollaston compared the oxides derived from both columbium—columbite, with a density 5.918 g/cm3, and tantalum—tantalite, with a density over 8 g/cm3, and concluded that the two oxides, despite the significant difference in density, were identical; thus he kept the name tantalum.[10] This conclusion was disputed in 1846 by German chemist Heinrich Rose, who argued that there were two different elements in the tantalite sample, and named them after children of Tantalus: niobium (from Niobe) and pelopium (from Pelops).[11][12] This confusion arose from the minimal observed differences between tantalum and niobium. The claimed new elements pelopium, ilmenium, and dianium[13] were in fact identical to niobium or mixtures of niobium and tantalum.[14]
The differences between tantalum and niobium were unequivocally demonstrated in 1864 by Christian Wilhelm Blomstrand[14] and Henri Etienne Sainte-Claire Deville, as well as Louis J. Troost, who determined the formulas of some of the compounds in 1865[14][15] and finally by Swiss chemist Jean Charles Galissard de Marignac[16] in 1866, who all proved that there were only two elements. Articles on ilmenium continued to appear until 1871.[17]
De Marignac was the first to prepare the metal in 1864, when he reduced niobium chloride by heating it in an atmosphere of hydrogen.[18] Although de Marignac was able to produce tantalum-free niobium on a larger scale by 1866, it was not until the early 20th century that niobium was used in incandescent lamp filaments, the first commercial application.[15] This use quickly became obsolete through the replacement of niobium with tungsten, which has a higher melting point. That niobium improves the strength of steel was first discovered in the 1920s, and this application remains its predominant use.[15] In 1961, the American physicist Eugene Kunzler and coworkers at Bell Labs discovered that niobium-tin continues to exhibit superconductivity in the presence of strong electric currents and magnetic fields,[19] making it the first material to support the high currents and fields necessary for useful high-power magnets and electrical power machinery. This discovery enabled – two decades later – the production of long multi-strand cables wound into coils to create large, powerful electromagnets for rotating machinery, particle accelerators, and particle detectors.[20][21]
Naming the element
Columbium (symbol "Cb")[22] was the name originally bestowed by Hatchett upon his discovery of the metal in 1801.[5] The name reflected that the type specimen of the ore came from America (Columbia).[23] This name remained in use in American journals—the last paper published by American Chemical Society with columbium in its title dates from 1953[24]—while niobium was used in Europe. To end this confusion, the name niobium was chosen for element 41 at the 15th Conference of the Union of Chemistry in Amsterdam in 1949.[25] A year later this name was officially adopted by the International Union of Pure and Applied Chemistry (IUPAC) after 100 years of controversy, despite the chronological precedence of the name columbium.[25] This was a compromise of sorts;[25] the IUPAC accepted tungsten instead of wolfram in deference to North American usage; and niobium instead of columbium in deference to European usage. While many US chemical societies and government organizations typically use the official IUPAC name, some metallurgists and metal societies still use the original American name, "columbium".[26][27][28][29]
Characteristics
Physical
Niobium is a lustrous, grey, ductile, paramagnetic metal in group 5 of the periodic table (see table), with an electron configuration in the outermost shells atypical for group 5. (This can be observed in the neighborhood of ruthenium (44), rhodium (45), and palladium (46).)
| Z | Element | No. of electrons/shell |
|---|---|---|
| 23 | vanadium | 2, 8, 11, 2 |
| 41 | niobium | 2, 8, 18, 12, 1 |
| 73 | tantalum | 2, 8, 18, 32, 11, 2 |
| 105 | dubnium | 2, 8, 18, 32, 32, 11, 2 |
Although it is thought to have a body-centered cubic crystal structure from absolute zero to its melting point, high-resolution measurements of the thermal expansion along the three crystallographic axes reveal anisotropies which are inconsistent with a cubic structure.[30] Therefore, further research and discovery in this area is expected.
Niobium becomes a superconductor at cryogenic temperatures. At atmospheric pressure, it has the highest critical temperature of the elemental superconductors at 9.2 K.[31] Niobium has the greatest magnetic penetration depth of any element.[31] In addition, it is one of the three elemental Type II superconductors, along with vanadium and technetium. The superconductive properties are strongly dependent on the purity of the niobium metal.[32]
When very pure, it is comparatively soft and ductile, but impurities make it harder.[33]
The metal has a low capture cross-section for thermal neutrons;[34] thus it is used in the nuclear industries where neutron transparent structures are desired.[35]
Chemical
The metal takes on a bluish tinge when exposed to air at room temperature for extended periods.[36] Despite a high melting point in elemental form (2,468 °C), it has a lower density than other refractory metals. Furthermore, it is corrosion-resistant, exhibits superconductivity properties, and forms dielectric oxide layers.
Niobium is slightly less electropositive and more compact than its predecessor in the periodic table, zirconium, whereas it is virtually identical in size to the heavier tantalum atoms, as a result of the lanthanide contraction.[33] As a result, niobium's chemical properties are very similar to those for tantalum, which appears directly below niobium in the periodic table.[15] Although its corrosion resistance is not as outstanding as that of tantalum, the lower price and greater availability make niobium attractive for less demanding applications, such as vat linings in chemical plants.[33]
Isotopes
Niobium in the Earth's crust comprises one stable isotope, 93Nb.[37] By 2003, at least 32 radioisotopes had been synthesized, ranging in atomic mass from 81 to 113. The most stable of these is 92Nb with a half-life of 34.7 million years. One of the least stable is 113Nb, with an estimated half-life of 30 milliseconds. Isotopes that are lighter than the stable 93Nb tend to decay by β+ decay, and those that are heavier tend to decay by β− decay, with some exceptions. 81Nb, 82Nb, and 84Nb have minor β+ delayed proton emission decay paths, 91Nb decays by electron capture and positron emission, and 92Nb decays by both β+ and β− decay.[37]
At least 25 nuclear isomers have been described, ranging in atomic mass from 84 to 104. Within this range, only 96Nb, 101Nb, and 103Nb do not have isomers. The most stable of niobium's isomers is 93mNb with a half-life of 16.13 years. The least stable isomer is 84mNb with a half-life of 103 ns. All of niobium's isomers decay by isomeric transition or beta decay except 92m1Nb, which has a minor electron capture branch.[37]
Occurrence
Niobium is estimated to be the 34th most common element in the Earth's crust, with 20 ppm.[38] Some think that the abundance on Earth is much greater, and that the element's high density has concentrated it in the Earth's core.[27] The free element is not found in nature, but niobium occurs in combination with other elements in minerals.[33] Minerals that contain niobium often also contain tantalum. Examples include columbite ((Fe,Mn)(Nb,Ta)2O6) and columbite–tantalite (or coltan, (Fe,Mn)(Ta,Nb)2O6).[39] Columbite–tantalite minerals (the most common species being columbite-(Fe) and tantalite-(Fe), where "-(Fe)" is the Levinson suffix informing about the prevailence of iron over other elements like manganese[40][41][42][43]) are most usually found as accessory minerals in pegmatite intrusions, and in alkaline intrusive rocks. Less common are the niobates of calcium, uranium, thorium and the rare earth elements. Examples of such niobates are pyrochlore ((Na,Ca)2Nb2O6(OH,F)) (now a group name, with a relatively common example being, e.g., fluorcalciopyrochlore[44][45][42][43][46]) and euxenite (correctly named euxenite-(Y)[47][42][43]) ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6). These large deposits of niobium have been found associated with carbonatites (carbonate-silicate igneous rocks) and as a constituent of pyrochlore.[48]
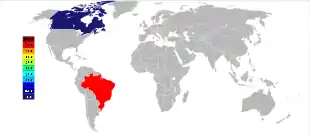
The three largest currently mined deposits of pyrochlore, two in Brazil and one in Canada, were found in the 1950s, and are still the major producers of niobium mineral concentrates.[15] The largest deposit is hosted within a carbonatite intrusion in Araxá, state of Minas Gerais, Brazil, owned by CBMM (Companhia Brasileira de Metalurgia e Mineração); the other active Brazilian deposit is located near Catalão, state of Goiás, and owned by China Molybdenum, also hosted within a carbonatite intrusion.[49] Together, those two mines produce about 88% of the world's supply.[50] Brazil also has a large but still unexploited deposit near São Gabriel da Cachoeira, state of Amazonas, as well as a few smaller deposits, notably in the state of Roraima.[50][51]
The third largest producer of niobium is the carbonatite-hosted Niobec mine, in Saint-Honoré, near Chicoutimi, Quebec, Canada, owned by Magris Resources.[52] It produces between 7% and 10% of the world's supply.[49][50]
Production
After the separation from the other minerals, the mixed oxides of tantalum Ta2O5 and niobium Nb2O5 are obtained. The first step in the processing is the reaction of the oxides with hydrofluoric acid:[39]
- Ta2O5 + 14 HF → 2 H2[TaF7] + 5 H2O
- Nb2O5 + 10 HF → 2 H2[NbOF5] + 3 H2O
The first industrial scale separation, developed by de Marignac, exploits the differing solubilities of the complex niobium and tantalum fluorides, dipotassium oxypentafluoroniobate monohydrate (K2[NbOF5]·H2O) and dipotassium heptafluorotantalate (K2[TaF7]) in water. Newer processes use the liquid extraction of the fluorides from aqueous solution by organic solvents like cyclohexanone.[39] The complex niobium and tantalum fluorides are extracted separately from the organic solvent with water and either precipitated by the addition of potassium fluoride to produce a potassium fluoride complex, or precipitated with ammonia as the pentoxide:[53]
- H2[NbOF5] + 2 KF → K2[NbOF5]↓ + 2 HF
Followed by:
- 2 H2[NbOF5] + 10 NH4OH → Nb2O5↓ + 10 NH4F + 7 H2O
Several methods are used for the reduction to metallic niobium. The electrolysis of a molten mixture of K2[NbOF5] and sodium chloride is one; the other is the reduction of the fluoride with sodium. With this method, a relatively high purity niobium can be obtained. In large scale production, Nb2O5 is reduced with hydrogen or carbon.[53] In the aluminothermic reaction, a mixture of iron oxide and niobium oxide is reacted with aluminium:
- 3 Nb2O5 + Fe2O3 + 12 Al → 6 Nb + 2 Fe + 6 Al2O3
Small amounts of oxidizers like sodium nitrate are added to enhance the reaction. The result is aluminium oxide and ferroniobium, an alloy of iron and niobium used in steel production.[54][55] Ferroniobium contains between 60 and 70% niobium.[49] Without iron oxide, the aluminothermic process is used to produce niobium. Further purification is necessary to reach the grade for superconductive alloys. Electron beam melting under vacuum is the method used by the two major distributors of niobium.[56][57]
As of 2013, CBMM from Brazil controlled 85 percent of the world's niobium production.[58] The United States Geological Survey estimates that the production increased from 38,700 tonnes in 2005 to 44,500 tonnes in 2006.[59][60] Worldwide resources are estimated to be 4,400,000 tonnes.[60] During the ten-year period between 1995 and 2005, the production more than doubled, starting from 17,800 tonnes in 1995.[61] Between 2009 and 2011, production was stable at 63,000 tonnes per year,[62] with a slight decrease in 2012 to only 50,000 tonnes per year.[63]
| Country | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 160 | 230 | 290 | 230 | 200 | 200 | 200 | ? | ? | ? | ? | ? | ? | ? | |
| 30,000 | 22,000 | 26,000 | 29,000 | 29,900 | 35,000 | 40,000 | 57,300 | 58,000 | 58,000 | 58,000 | 58,000 | 45,000 | 53,100 | |
| 2,290 | 3,200 | 3,410 | 3,280 | 3,400 | 3,310 | 4,167 | 3,020 | 4,380 | 4,330 | 4,420 | 4,630 | 4,710 | 5,260 | |
| ? | 50 | 50 | 13 | 52 | 25 | ? | ? | ? | ? | ? | ? | ? | ? | |
| ? | ? | 5 | 34 | 130 | 34 | 29 | ? | ? | ? | ? | ? | ? | ? | |
| 35 | 30 | 30 | 190 | 170 | 40 | 35 | ? | ? | ? | ? | ? | ? | ? | |
| 28 | 120 | 76 | 22 | 63 | 63 | 80 | ? | ? | ? | ? | ? | ? | ? | |
| World | 32,600 | 25,600 | 29,900 | 32,800 | 34,000 | 38,700 | 44,500 | 60,400 | 62,900 | 62,900 | 62,900 | 63,000 | 50,100 | 59,400 |
Lesser amounts are found in Malawi's Kanyika Deposit (Kanyika mine).
Compounds
In many ways, niobium is similar to tantalum and zirconium. It reacts with most nonmetals at high temperatures; with fluorine at room temperature; with chlorine at 150 °C and hydrogen at 200 °C; and with nitrogen at 400 °C, with products that are frequently interstitial and nonstoichiometric.[33] The metal begins to oxidize in air at 200 °C.[53] It resists corrosion by fused alkalis and by acids, including aqua regia, hydrochloric, sulfuric, nitric and phosphoric acids.[33] Niobium is attacked by hydrofluoric acid and hydrofluoric/nitric acid mixtures.
Although niobium exhibits all of the formal oxidation states from +5 to −1, the most common compounds have niobium in the +5 state.[33] Characteristically, compounds in oxidation states less than 5+ display Nb–Nb bonding. In aqueous solutions, niobium only exhibit the +5 oxidation state. It is also readily prone to hydrolysis and is barely soluble in dilute solutions of hydrochloric, sulfuric, nitric and phosphoric acids due to the precipitation of hydrous Nb oxide.[56] Nb(V) is also slightly soluble in alkaline media due to the formation of soluble polyoxoniobate species.[65][66]
Oxides and sulfides
Niobium forms oxides in the oxidation states +5 (Nb2O5),[67] +4 (NbO2), +3 (Nb
2O
3),[53] and the rarer oxidation state, +2 (NbO).[68] Most common is the pentoxide, precursor to almost all niobium compounds and alloys.[53][69] Niobates are generated by dissolving the pentoxide in basic hydroxide solutions or by melting it in alkali metal oxides. Examples are lithium niobate (LiNbO3) and lanthanum niobate (LaNbO4). In the lithium niobate is a trigonally distorted perovskite-like structure, whereas the lanthanum niobate contains lone NbO3−
4 ions.[53] The layered niobium sulfide (NbS2) is also known.[33]
Materials can be coated with a thin film of niobium(V) oxide chemical vapor deposition or atomic layer deposition processes, produced by the thermal decomposition of niobium(V) ethoxide above 350 °C.[70][71]
Halides

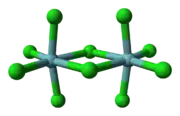
Niobium forms halides in the oxidation states of +5 and +4 as well as diverse substoichiometric compounds.[53][56] The pentahalides (NbX
5) feature octahedral Nb centres. Niobium pentafluoride (NbF5) is a white solid with a melting point of 79.0 °C and niobium pentachloride (NbCl5) is yellow (see image at left) with a melting point of 203.4 °C. Both are hydrolyzed to give oxides and oxyhalides, such as NbOCl3. The pentachloride is a versatile reagent used to generate the organometallic compounds, such as niobocene dichloride ((C
5H
5)
2NbCl
2).[72] The tetrahalides (NbX
4) are dark-coloured polymers with Nb-Nb bonds; for example, the black hygroscopic niobium tetrafluoride (NbF4) and brown niobium tetrachloride (NbCl4).
Anionic halide compounds of niobium are well known, owing in part to the Lewis acidity of the pentahalides. The most important is [NbF7]2−, an intermediate in the separation of Nb and Ta from the ores.[39] This heptafluoride tends to form the oxopentafluoride more readily than does the tantalum compound. Other halide complexes include octahedral [NbCl6]−:
- Nb2Cl10 + 2 Cl− → 2 [NbCl6]−
As with other metals with low atomic numbers, a variety of reduced halide cluster ions is known, the prime example being [Nb6Cl18]4−.[73]
Nitrides and carbides
Other binary compounds of niobium include niobium nitride (NbN), which becomes a superconductor at low temperatures and is used in detectors for infrared light.[74] The main niobium carbide is NbC, an extremely hard, refractory, ceramic material, commercially used in cutting tool bits.
Applications
Out of 44,500 tonnes of niobium mined in 2006, an estimated 90% was used in high-grade structural steel. The second largest application is superalloys.[75] Niobium alloy superconductors and electronic components account for a very small share of the world production.[75]
Steel production
Niobium is an effective microalloying element for steel, within which it forms niobium carbide and niobium nitride.[27] These compounds improve the grain refining, and retard recrystallization and precipitation hardening. These effects in turn increase the toughness, strength, formability, and weldability.[27] Within microalloyed stainless steels, the niobium content is a small (less than 0.1%[76]) but important addition to high strength low alloy steels that are widely used structurally in modern automobiles.[27] Niobium is sometimes used in considerably higher quantities for highly wear-resistant machine components and knives, as high as 3% in Crucible CPM S110V stainless steel.[77]
These same niobium alloys are often used in pipeline construction.[78][79]
Superalloys
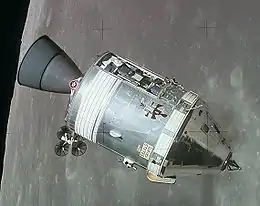
Quantities of niobium are used in nickel-, cobalt-, and iron-based superalloys in proportions as great as 6.5%[76] for such applications as jet engine components, gas turbines, rocket subassemblies, turbo charger systems, heat resisting, and combustion equipment. Niobium precipitates a hardening γ''-phase within the grain structure of the superalloy.[80]
One example superalloy is Inconel 718, consisting of roughly 50% nickel, 18.6% chromium, 18.5% iron, 5% niobium, 3.1% molybdenum, 0.9% titanium, and 0.4% aluminium.[81][82] These superalloys were used, for example, in advanced air frame systems for the Gemini program. Another niobium alloy was used for the nozzle of the Apollo Service Module. Because niobium is oxidized at temperatures above 400 °C, a protective coating is necessary for these applications to prevent the alloy from becoming brittle.[83]
Niobium-based alloys
C-103 alloy was developed in the early 1960s jointly by the Wah Chang Corporation and Boeing Co. DuPont, Union Carbide Corp., General Electric Co. and several other companies were developing Nb-base alloys simultaneously, largely driven by the Cold War and Space Race. It is composed of 89% niobium, 10% hafnium and 1% titanium and is used for liquid rocket thruster nozzles, such as the main engine of the Apollo Lunar Modules.[83]

The nozzle of the Merlin Vacuum series of engines developed by SpaceX for the upper stage of its Falcon 9 rocket is made from a niobium alloy.[84]
The reactivity of niobium with oxygen requires it to be worked in a vacuum or inert atmosphere, which significantly increases the cost and difficulty of production. Vacuum arc remelting (VAR) and electron beam melting (EBM), novel processes at the time, enabled the development of niobium and other reactive metals. The project that yielded C-103 began in 1959 with as many as 256 experimental niobium alloys in the "C-series" (possibly from columbium) that could be melted as buttons and rolled into sheet. Wah Chang had an inventory of hafnium, refined from nuclear-grade zirconium alloys, that it wanted to put to commercial use. The 103rd experimental composition of the C-series alloys, Nb-10Hf-1Ti, had the best combination of formability and high-temperature properties. Wah Chang fabricated the first 500-lb heat of C-103 in 1961, ingot to sheet, using EBM and VAR. The intended applications included turbine engines and liquid metal heat exchangers. Competing niobium alloys from that era included FS85 (Nb-10W-28Ta-1Zr) from Fansteel Metallurgical Corp., Cb129Y (Nb-10W-10Hf-0.2Y) from Wah Chang and Boeing, Cb752 (Nb-10W-2.5Zr) from Union Carbide, and Nb1Zr from Superior Tube Co.[83]
Superconducting magnets
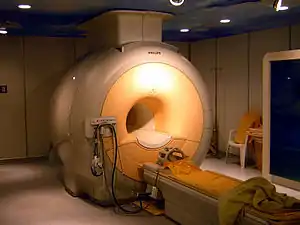
Niobium-germanium (Nb
3Ge), niobium-tin (Nb
3Sn), as well as the niobium-titanium alloys are used as a type II superconductor wire for superconducting magnets.[85][86] These superconducting magnets are used in magnetic resonance imaging and nuclear magnetic resonance instruments as well as in particle accelerators.[87] For example, the Large Hadron Collider uses 600 tons of superconducting strands, while the International Thermonuclear Experimental Reactor uses an estimated 600 tonnes of Nb3Sn strands and 250 tonnes of NbTi strands.[88] In 1992 alone, more than US$1 billion worth of clinical magnetic resonance imaging systems were constructed with niobium-titanium wire.[20]
Other superconductors

The superconducting radio frequency (SRF) cavities used in the free-electron lasers FLASH (result of the cancelled TESLA linear accelerator project) and XFEL are made from pure niobium.[89] A cryomodule team at Fermilab used the same SRF technology from the FLASH project to develop 1.3 GHz nine-cell SRF cavities made from pure niobium. The cavities will be used in the 30-kilometre (19 mi) linear particle accelerator of the International Linear Collider.[90] The same technology will be used in LCLS-II at SLAC National Accelerator Laboratory and PIP-II at Fermilab.[91]
The high sensitivity of superconducting niobium nitride bolometers make them an ideal detector for electromagnetic radiation in the THz frequency band. These detectors were tested at the Submillimeter Telescope, the South Pole Telescope, the Receiver Lab Telescope, and at APEX, and are now used in the HIFI instrument on board the Herschel Space Observatory.[92]
Electroceramics
Lithium niobate, which is a ferroelectric, is used extensively in mobile telephones and optical modulators, and for the manufacture of surface acoustic wave devices. It belongs to the ABO3 structure ferroelectrics like lithium tantalate and barium titanate.[93] Niobium capacitors are available as alternative to tantalum capacitors,[94] but tantalum capacitors still predominate. Niobium is added to glass to obtain a higher refractive index, making possible thinner and lighter corrective glasses.
Hypoallergenic applications: medicine and jewelry
Niobium and some niobium alloys are physiologically inert and hypoallergenic. For this reason, niobium is used in prosthetics and implant devices, such as pacemakers.[95] Niobium treated with sodium hydroxide forms a porous layer that aids osseointegration.[96]
Like titanium, tantalum, and aluminium, niobium can be heated and anodized ("reactive metal anodization") to produce a wide array of iridescent colours for jewelry,[97][98] where its hypoallergenic property is highly desirable.[99]
Numismatics

Niobium is used as a precious metal in commemorative coins, often with silver or gold. For example, Austria produced a series of silver niobium euro coins starting in 2003; the colour in these coins is created by the diffraction of light by a thin anodized oxide layer.[100] In 2012, ten coins are available showing a broad variety of colours in the centre of the coin: blue, green, brown, purple, violet, or yellow. Two more examples are the 2004 Austrian €25 150 Years Semmering Alpine Railway commemorative coin,[101] and the 2006 Austrian €25 European Satellite Navigation commemorative coin.[102] The Austrian mint produced for Latvia a similar series of coins starting in 2004,[103] with one following in 2007.[104] In 2011, the Royal Canadian Mint started production of a $5 sterling silver and niobium coin named Hunter's Moon[105] in which the niobium was selectively oxidized, thus creating unique finishes where no two coins are exactly alike.
Other
The arc-tube seals of high pressure sodium vapor lamps are made from niobium, sometimes alloyed with 1% of zirconium; niobium has a very similar coefficient of thermal expansion, matching the sintered alumina arc tube ceramic, a translucent material which resists chemical attack or reduction by the hot liquid sodium and sodium vapour contained inside the operating lamp.[106][107][108]
Niobium is used in arc welding rods for some stabilized grades of stainless steel[109] and in anodes for cathodic protection systems on some water tanks, which are then usually plated with platinum.[110][111]
Niobium is an important component of high-performance heterogeneous catalysts for the production of acrylic acid by selective oxidation of propane.[112][113][114][115]
Niobium is used to make the high voltage wire of the solar corona particles receptor module of the Parker Solar Probe.[116]
Precautions
| Hazards | |
|---|---|
| NFPA 704 (fire diamond) | |
Niobium has no known biological role. While niobium dust is an eye and skin irritant and a potential fire hazard, elemental niobium on a larger scale is physiologically inert (and thus hypoallergenic) and harmless. It is frequently used in jewelry and has been tested for use in some medical implants.[117][118]
Niobium-containing compounds are rarely encountered by most people, but some are toxic and should be treated with care. The short- and long-term exposure to niobates and niobium chloride, two chemicals that are water-soluble, have been tested in rats. Rats treated with a single injection of niobium pentachloride or niobates show a median lethal dose (LD50) between 10 and 100 mg/kg.[119][120][121] For oral administration the toxicity is lower; a study with rats yielded a LD50 after seven days of 940 mg/kg.[119]
References
- Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- G.V. Samsonov, ed. (1968). "Mechanical Properties of the Elements". Handbook of the physicochemical properties of the elements. New York, USA: IFI-Plenum. pp. 387–446. doi:10.1007/978-1-4684-6066-7_7. ISBN 978-1-4684-6066-7. Archived from the original on 2 April 2015.
- Knapp, Brian (2002). Francium to Polonium. Atlantic Europe Publishing Company, p. 40. ISBN 0717256774.
- Hatchett, Charles (1802). "An analysis of a mineral substance from North America, containing a metal hitherto unknown". Philosophical Transactions of the Royal Society of London. 92: 49–66. doi:10.1098/rspl.1800.0045. JSTOR 107114.
- Hatchett, Charles (1802), "Outline of the Properties and Habitudes of the Metallic Substance, lately discovered by Charles Hatchett, Esq. and by him denominated Columbium", Journal of Natural Philosophy, Chemistry, and the Arts, I (January): 32–34.
- Hatchett, Charles (1802). "Eigenschaften und chemisches Verhalten des von Charles Hatchett entdeckten neuen Metalls, Columbium" [Properties and chemical behavior of the new metal, columbium, (that was) discovered by Charles Hatchett]. Annalen der Physik (in German). 11 (5): 120–122. Bibcode:1802AnP....11..120H. doi:10.1002/andp.18020110507.
- Noyes, William Albert (1918). A Textbook of Chemistry. H. Holt & Co. p. 523.
- Percival, James (January 1853). "Middletown Silver and Lead Mines". Journal of Silver and Lead Mining Operations. 1: 186. Retrieved 24 April 2013.
- Griffith, William P.; Morris, Peter J. T. (2003). "Charles Hatchett FRS (1765–1847), Chemist and Discoverer of Niobium". Notes and Records of the Royal Society of London. 57 (3): 299–316. doi:10.1098/rsnr.2003.0216. JSTOR 3557720. S2CID 144857368.
- Wollaston, William Hyde (1809). "On the Identity of Columbium and Tantalum". Philosophical Transactions of the Royal Society. 99: 246–252. doi:10.1098/rstl.1809.0017. JSTOR 107264. S2CID 110567235.
- Rose, Heinrich (1844). "Ueber die Zusammensetzung der Tantalite und ein im Tantalite von Baiern enthaltenes neues Metall". Annalen der Physik (in German). 139 (10): 317–341. Bibcode:1844AnP...139..317R. doi:10.1002/andp.18441391006.
- Rose, Heinrich (1847). "Ueber die Säure im Columbit von Nordamérika". Annalen der Physik (in German). 146 (4): 572–577. Bibcode:1847AnP...146..572R. doi:10.1002/andp.18471460410.
- Kobell, V. (1860). "Ueber eine eigenthümliche Säure, Diansäure, in der Gruppe der Tantal- und Niob- verbindungen". Journal für Praktische Chemie. 79 (1): 291–303. doi:10.1002/prac.18600790145.
- Marignac, Blomstrand; Deville, H.; Troost, L.; Hermann, R. (1866). "Tantalsäure, Niobsäure, (Ilmensäure) und Titansäure". Fresenius' Journal of Analytical Chemistry. 5 (1): 384–389. doi:10.1007/BF01302537. S2CID 97246260.
- Gupta, C. K.; Suri, A. K. (1994). Extractive Metallurgy of Niobium. CRC Press. pp. 1–16. ISBN 978-0-8493-6071-8.
- Marignac, M. C. (1866). "Recherches sur les combinaisons du niobium". Annales de chimie et de physique (in French). 4 (8): 7–75.
- Hermann, R. (1871). "Fortgesetzte Untersuchungen über die Verbindungen von Ilmenium und Niobium, sowie über die Zusammensetzung der Niobmineralien (Further research about the compounds of ilmenium and niobium, as well as the composition of niobium minerals)". Journal für Praktische Chemie (in German). 3 (1): 373–427. doi:10.1002/prac.18710030137.
- "Niobium". Universidade de Coimbra. Archived from the original on 10 December 2007. Retrieved 5 September 2008.
- Geballe et al. (1993) gives a critical point at currents of 150 kiloamperes and magnetic fields of 8.8 tesla.
- Geballe, Theodore H. (October 1993). "Superconductivity: From Physics to Technology". Physics Today. 46 (10): 52–56. Bibcode:1993PhT....46j..52G. doi:10.1063/1.881384.
- Matthias, B. T.; Geballe, T. H.; Geller, S.; Corenzwit, E. (1954). "Superconductivity of Nb3Sn". Physical Review. 95 (6): 1435. Bibcode:1954PhRv...95.1435M. doi:10.1103/PhysRev.95.1435.
- Kòrösy, F. (1939). "Reaction of Tantalum, Columbium and Vanadium with Iodine". Journal of the American Chemical Society. 61 (4): 838–843. doi:10.1021/ja01873a018.
- Nicholson, William, ed. (1809), The British Encyclopedia: Or, Dictionary of Arts and Sciences, Comprising an Accurate and Popular View of the Present Improved State of Human Knowledge, 2, Longman, Hurst, Rees, and Orme, p. 284.
- Ikenberry, L.; Martin, J. L.; Boyer, W. J. (1953). "Photometric Determination of Columbium, Tungsten, and Tantalum in Stainless Steels". Analytical Chemistry. 25 (9): 1340–1344. doi:10.1021/ac60081a011.
- Rayner-Canham, Geoff; Zheng, Zheng (2008). "Naming elements after scientists: an account of a controversy". Foundations of Chemistry. 10 (1): 13–18. doi:10.1007/s10698-007-9042-1. S2CID 96082444.
- Clarke, F. W. (1914). "Columbium Versus Niobium". Science. 39 (995): 139–140. Bibcode:1914Sci....39..139C. doi:10.1126/science.39.995.139. JSTOR 1640945. PMID 17780662.
- Patel, Zh.; Khul'ka K. (2001). "Niobium for Steelmaking". Metallurgist. 45 (11–12): 477–480. doi:10.1023/A:1014897029026. S2CID 137569464.
- Norman N., Greenwood (2003). "Vanadium to dubnium: from confusion through clarity to complexity". Catalysis Today. 78 (1–4): 5–11. doi:10.1016/S0920-5861(02)00318-8.
- "ASTM A572 / A572M-18, Standard Specification for High-Strength Low-Alloy Columbium-Vanadium Structural Steel". ASTM International, West Conshohocken. 2018. Retrieved 12 February 2020.
- Bollinger, R. K.; White, B. D.; Neumeier, J. J.; Sandim, H. R. Z.; Suzuki, Y.; dos Santos, C. A. M.; Avci, R.; Migliori, A.; Betts, J. B. (2011). "Observation of a Martensitic Structural Distortion in V, Nb, and Ta". Physical Review Letters. 107 (7): 075503. Bibcode:2011PhRvL.107g5503B. doi:10.1103/PhysRevLett.107.075503. PMID 21902404.
- Peiniger, M.; Piel, H. (1985). "A Superconducting Nb3Sn Coated Multicell Accelerating Cavity". IEEE Transactions on Nuclear Science. 32 (5): 3610–3612. Bibcode:1985ITNS...32.3610P. doi:10.1109/TNS.1985.4334443. S2CID 23988671.
- Salles Moura, Hernane R.; Louremjo de Moura, Louremjo (2007). "Melting And Purification of Niobium". AIP Conference Proceedings. 927 (927): 165–178. Bibcode:2007AIPC..927..165M. doi:10.1063/1.2770689.
- Nowak, Izabela; Ziolek, Maria (1999). "Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis". Chemical Reviews. 99 (12): 3603–3624. doi:10.1021/cr9800208. PMID 11849031.
- Jahnke, L. P.; Frank, R. G.; Redden, T. K. (1960). "Columbium Alloys Today". Metal Progr. 77 (6): 69–74. OSTI 4183692.
- Nikulina, A. V. (2003). "Zirconium-Niobium Alloys for Core Elements of Pressurized Water Reactors". Metal Science and Heat Treatment. 45 (7–8): 287–292. Bibcode:2003MSHT...45..287N. doi:10.1023/A:1027388503837. S2CID 134841512.
- Lide, David R. (2004). "The Elements". CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. pp. 4–21. ISBN 978-0-8493-0485-9.
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- Emsley, John (2001). "Niobium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England: Oxford University Press. pp. 283–286. ISBN 978-0-19-850340-8.
- Soisson, Donald J.; McLafferty, J. J.; Pierret, James A. (1961). "Staff-Industry Collaborative Report: Tantalum and Niobium". Industrial and Engineering Chemistry. 53 (11): 861–868. doi:10.1021/ie50623a016.
- "Columbite-(Fe): Mineral information, data and localities". www.mindat.org.
- "Tantalite-(Fe): Mineral information, data and localities". www.mindat.org.
- Burke, Ernst A.J. (2008). "The use of suffixes in mineral names" (PDF). Elements. 4 (2): 96. Retrieved 7 December 2019.
- "CNMNC". nrmima.nrm.se. Archived from the original on 10 August 2019. Retrieved 6 October 2018.
- "Pyrochlore Group: Mineral information, data and localities". www.mindat.org.
- "Fluorcalciopyrochlore: Mineral information, data and localities". www.mindat.org.
- http://rruff.info/uploads/AM62_403.pdf
- "Euxenite-(Y): Mineral information, data and localities". www.mindat.org.
- Lumpkin, Gregory R.; Ewing, Rodney C. (1995). "Geochemical alteration of pyrochlore group minerals: Pyrochlore subgroup" (PDF). American Mineralogist. 80 (7–8): 732–743. Bibcode:1995AmMin..80..732L. doi:10.2138/am-1995-7-810.
- Kouptsidis, J.; Peters, F.; Proch, D.; Singer, W. "Niob für TESLA" (PDF) (in German). Deutsches Elektronen-Synchrotron DESY. Archived from the original (PDF) on 17 December 2008. Retrieved 2 September 2008.
- Alvarenga, Darlan (9 April 2013). "'Monopólio' brasileiro do nióbio gera cobiça mundial, controvérsia e mitos" [Brazilian niobium 'monopoly' brings about the world's greed, controversy, and myths]. G1 (in Portuguese). São Paulo. Retrieved 23 May 2016.
- Siqueira-Gay, Juliana; Sánchez, Luis E. (2020). "Keep the Amazon niobium in the ground". Environmental Science & Policy. 111: 1–6. doi:10.1016/j.envsci.2020.05.012. ISSN 1462-9011.
- "Magris Resources, officially owner of Niobec" (Press release). Niobec. 23 January 2015. Retrieved 23 May 2016.
- Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Niob". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1075–1079. ISBN 978-3-11-007511-3.
- Tither, Geoffrey (2001). Minerals, Metals and Materials Society (ed.). Progress in Niobium Markets and Technology 1981–2001 (PDF). Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001 (Orlando, Florida, USA). ISBN 978-0-9712068-0-9. Archived from the original (PDF) on 17 December 2008.
- Dufresne, Claude; Goyette, Ghislain (2001). Minerals, Metals and Materials Society (ed.). The Production of Ferroniobium at the Niobec mine 1981–2001 (PDF). Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001 (Orlando, Florida, USA). ISBN 978-0-9712068-0-9. Archived from the original (PDF) on 17 December 2008.
- Agulyansky, Anatoly (2004). The Chemistry of Tantalum and Niobium Fluoride Compounds. Elsevier. pp. 1–11. ISBN 978-0-444-51604-6.
- Choudhury, Alok; Hengsberger, Eckart (1992). "Electron Beam Melting and Refining of Metals and Alloys". The Iron and Steel Institute of Japan International. 32 (5): 673–681. doi:10.2355/isijinternational.32.673.
- Lucchesi, Cristane; Cuadros, Alex (April 2013), "Mineral Wealth", Bloomberg Markets (paper), p. 14
- Papp, John F. "Niobium (Columbium)" (PDF). USGS 2006 Commodity Summary. Retrieved 20 November 2008.
- Papp, John F. "Niobium (Columbium)" (PDF). USGS 2007 Commodity Summary. Retrieved 20 November 2008.
- Papp, John F. "Niobium (Columbium)" (PDF). USGS 1997 Commodity Summary. Retrieved 20 November 2008.
- Niobium (Colombium) U.S. Geological Survey, Mineral Commodity Summaries, January 2011
- Niobium (Colombium) U.S. Geological Survey, Mineral Commodity Summaries, January 2016
- Cunningham, Larry D. (5 April 2012). "USGS Minerals Information: Niobium (Columbium) and Tantalum". Minerals.usgs.gov. Retrieved 17 August 2012.
- Deblonde, Gauthier J. -P.; Chagnes, Alexandre; Bélair, Sarah; Cote, Gérard (1 July 2015). "Solubility of niobium(V) and tantalum(V) under mild alkaline conditions". Hydrometallurgy. 156: 99–106. doi:10.1016/j.hydromet.2015.05.015. ISSN 0304-386X.
- Nyman, May (2 August 2011). "Polyoxoniobate chemistry in the 21st century". Dalton Transactions. 40 (32): 8049–8058. doi:10.1039/C1DT10435G. ISSN 1477-9234. PMID 21670824.
- Pubchem. "Niobium oxide | Nb2O5 – PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 29 June 2016.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Cardarelli, Francois (2008). Materials Handbook. Springer London. ISBN 978-1-84628-668-1.
- Rahtu, Antti (2002). Atomic Layer Deposition of High Permittivity Oxides: Film Growth and In Situ Studies (Thesis). University of Helsinki. hdl:10138/21065. ISBN 952-10-0646-3.
- Maruyama, Toshiro (1994). "Electrochromic Properties of Niobium Oxide Thin Films Prepared by Chemical Vapor Deposition". Journal of the Electrochemical Society. 141 (10): 2868–2871. doi:10.1149/1.2059247.
- Lucas, C. R.; Labinger, J. A.; Schwartz, J. (1990). Robert J. Angelici (ed.). Dichlorobis(η5-Cyclopentadienyl)Niobium(IV). Inorganic Syntheses. 28. New York. pp. 267–270. doi:10.1002/9780470132593.ch68. ISBN 978-0-471-52619-3.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Verevkin, A.; Pearlman, A.; Slstrokysz, W.; Zhang, J.; et al. (2004). "Ultrafast superconducting single-photon detectors for near-infrared-wavelength quantum communications". Journal of Modern Optics. 51 (12): 1447–1458. doi:10.1080/09500340410001670866.
- Papp, John F. "Niobium (Columbium ) and Tantalum" (PDF). USGS 2006 Minerals Yearbook. Retrieved 3 September 2008.
- Heisterkamp, Friedrich; Carneiro, Tadeu (2001). Minerals, Metals and Materials Society (ed.). Niobium: Future Possibilities – Technology and the Market Place (PDF). Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001 (Orlando, Florida, USA). ISBN 978-0-9712068-0-9. Archived from the original (PDF) on 17 December 2008.
- "Datasheet CPM S110V" (PDF). Crucible Industries LLC. Retrieved 20 November 2017.
- Eggert, Peter; Priem, Joachim; Wettig, Eberhard (1982). "Niobium: a steel additive with a future". Economic Bulletin. 19 (9): 8–11. doi:10.1007/BF02227064. S2CID 153775645.
- Hillenbrand, Hans-Georg; Gräf, Michael; Kalwa, Christoph (2 May 2001). "Development and Production of High Strength Pipeline Steels" (PDF). Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001 (Orlando, Florida, USA). Archived from the original (PDF) on 5 June 2015.
- Donachie, Matthew J. (2002). Superalloys: A Technical Guide. ASM International. pp. 29–30. ISBN 978-0-87170-749-9.
- Bhadeshia, H. k. d. h. "Nickel Based Superalloys". University of Cambridge. Archived from the original on 25 August 2006. Retrieved 4 September 2008.
- Pottlacher, G.; Hosaeus, H.; Wilthan, B.; Kaschnitz, E.; Seifter, A. (2002). "Thermophysikalische Eigenschaften von festem und flüssigem Inconel 718". Thermochimica Acta (in German). 382 (1––2): 55–267. doi:10.1016/S0040-6031(01)00751-1.
- Hebda, John (2 May 2001). "Niobium alloys and high Temperature Applications" (PDF). Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001 (Orlando, Florida, USA). Archived from the original (PDF) on 17 December 2008.
- Dinardi, Aaron; Capozzoli, Peter; Shotwell, Gwynne (2008). Low-cost Launch Opportunities Provided by the Falcon Family of Launch Vehicles (PDF). Fourth Asian Space Conference. Taipei. Archived from the original (PDF) on 15 March 2012.
- Lindenhovius, J.L.H.; Hornsveld, E. M.; Den Ouden, A.; Wessel, W. A. J.; et al. (2000). "Powder-in-tube (PIT) Nb/sub 3/Sn conductors for high-field magnets". IEEE Transactions on Applied Superconductivity. 10 (1): 975–978. Bibcode:2000ITAS...10..975L. doi:10.1109/77.828394. S2CID 26260700.
- Nave, Carl R. "Superconducting Magnets". Georgia State University, Department of Physics and Astronomy. Retrieved 25 November 2008.
- Glowacki, B. A.; Yan, X. -Y.; Fray, D.; Chen, G.; Majoros, M.; Shi, Y. (2002). "Niobium based intermetallics as a source of high-current/high magnetic field superconductors". Physica C: Superconductivity. 372–376 (3): 1315–1320. arXiv:cond-mat/0109088. Bibcode:2002PhyC..372.1315G. doi:10.1016/S0921-4534(02)01018-3. S2CID 118990555.
- Grunblatt, G.; Mocaer, P.; Verwaerde Ch.; Kohler, C. (2005). "A success story: LHC cable production at ALSTOM-MSA". Fusion Engineering and Design (Proceedings of the 23rd Symposium of Fusion Technology). 75–79 (2): 3516. Bibcode:2005ITAS...15.3516M. doi:10.1016/j.fusengdes.2005.06.216. S2CID 41810761.
- Lilje, L.; Kako, E.; Kostin, D.; Matheisen, A.; et al. (2004). "Achievement of 35 MV/m in the superconducting nine-cell cavities for TESLA". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 524 (1–3): 1–12. arXiv:physics/0401141. Bibcode:2004NIMPA.524....1L. doi:10.1016/j.nima.2004.01.045. S2CID 2141809.
- The International Linear Collider Technical Design Report 2013. International Linear Collider. 2013. Retrieved 15 August 2015.
- "ILC-type cryomodule makes the grade". CERN Courier. IOP Publishing. 27 November 2014. Retrieved 15 August 2015.
- Cherednichenko, Sergey; Drakinskiy, Vladimir; Berg, Therese; Khosropanah, Pourya; et al. (2008). "A Hot-electron bolometer terahertz mixers for the Herschel Space Observatory". Review of Scientific Instruments. 79 (3): 0345011–03451010. Bibcode:2008RScI...79c4501C. doi:10.1063/1.2890099. PMID 18377032.
- Volk, Tatyana; Wohlecke, Manfred (2008). Lithium Niobate: Defects, Photorefraction and Ferroelectric Switching. Springer. pp. 1–9. ISBN 978-3-540-70765-3.
- Pozdeev, Y. (1991). "Reliability comparison of tantalum and niobium solid electrolytic capacitors". Quality and Reliability Engineering International. 14 (2): 79–82. doi:10.1002/(SICI)1099-1638(199803/04)14:2<79::AID-QRE163>3.0.CO;2-Y.
- Mallela, Venkateswara Sarma; Ilankumaran, V.; Srinivasa Rao, N. (1 January 2004). "Trends in Cardiac Pacemaker Batteries". Indian Pacing Electrophysiol J. 4 (4): 201–212. PMC 1502062. PMID 16943934.
- Godley, Reut; Starosvetsky, David; Gotman, Irena (2004). "Bonelike apatite formation on niobium metal treated in aqueous NaOH". Journal of Materials Science: Materials in Medicine. 15 (10): 1073–1077. doi:10.1023/B:JMSM.0000046388.07961.81. PMID 15516867. S2CID 44988090.
- Biason Gomes, M. A.; Onofre, S.; Juanto, S.; Bulhões, L. O. de S. (1991). "Anodization of niobium in sulphuric acid media". Journal of Applied Electrochemistry. 21 (11): 1023–1026. doi:10.1007/BF01077589. S2CID 95285286.
- Chiou, Y. L. (1971). "A note on the thicknesses of anodized niobium oxide films". Thin Solid Films. 8 (4): R37–R39. Bibcode:1971TSF.....8R..37C. doi:10.1016/0040-6090(71)90027-7.
- Azevedo, C. R. F.; Spera, G.; Silva, A. P. (2002). "Characterization of metallic piercings that caused adverse reactions during use". Journal of Failure Analysis and Prevention. 2 (4): 47–53. doi:10.1361/152981502770351860.
- Grill, Robert; Gnadenberge, Alfred (2006). "Niobium as mint metal: Production–properties–processing". International Journal of Refractory Metals and Hard Materials. 24 (4): 275–282. doi:10.1016/j.ijrmhm.2005.10.008.
- "25 Euro – 150 Years Semmering Alpine Railway (2004)". Austrian Mint. Archived from the original on 21 July 2011. Retrieved 4 November 2008.
- "150 Jahre Semmeringbahn" (in German). Austrian Mint. Archived from the original on 20 July 2011. Retrieved 4 September 2008.
- "Neraža – mēs nevarējām atrast meklēto lapu!" (in Latvian). Bank of Latvia. Archived from the original on 9 January 2008. Retrieved 19 September 2008.
- "Neraža – mēs nevarējām atrast meklēto lapu!" (in Latvian). Bank of Latvia. Archived from the original on 22 May 2009. Retrieved 19 September 2008.
- "$5 Sterling Silver and Niobium Coin – Hunter's Moon (2011)". Royal Canadian Mint. Retrieved 1 February 2012.
- Henderson, Stanley Thomas; Marsden, Alfred Michael; Hewitt, Harry (1972). Lamps and Lighting. Edward Arnold Press. pp. 244–245. ISBN 978-0-7131-3267-0.
- Eichelbrönner, G. (1998). "Refractory metals: crucial components for light sources". International Journal of Refractory Metals and Hard Materials. 16 (1): 5–11. doi:10.1016/S0263-4368(98)00009-2.
- Michaluk, Christopher A.; Huber, Louis E.; Ford, Robert B. (2001). Minerals, Metals and Materials Society (ed.). Niobium and Niobium 1% Zirconium for High Pressure Sodium (HPS) Discharge Lamps. Niobium Science & Technology: Proceedings of the International Symposium Niobium 2001 (Orlando, Florida, USA). ISBN 978-0-9712068-0-9.
- US patent 5254836, Okada, Yuuji; Kobayashi, Toshihiko; Sasabe, Hiroshi; Aoki, Yoshimitsu; Nishizawa, Makoto; Endo, Shunji, "Method of arc welding with a ferrite stainless steel welding rod", issued 19 October 1993
- Moavenzadeh, Fred (14 March 1990). Concise Encyclopedia of Building and Construction Materials. MIT Press. pp. 157–. ISBN 978-0-262-13248-0. Retrieved 18 February 2012.
- Cardarelli, François (9 January 2008). Materials handbook: a concise desktop reference. Springer. pp. 352–. ISBN 978-1-84628-668-1. Retrieved 18 February 2012.
- Hävecker, Michael; Wrabetz, Sabine; Kröhnert, Jutta; Csepei, Lenard-Istvan; Naumann d'Alnoncourt, Raoul; Kolen'Ko, Yury V; Girgsdies, Frank; Schlögl, Robert; Trunschke, Annette (2012). "Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid" (PDF). Journal of Catalysis. 285: 48–60. doi:10.1016/j.jcat.2011.09.012. hdl:11858/00-001M-0000-0012-1BEB-F.
- Amakawa, Kazuhiko; Kolen'Ko, Yury V; Villa, Alberto; Schuster, Manfred E/; Csepei, Lénárd-István; Weinberg, Gisela; Wrabetz, Sabine; Naumann d'Alnoncourt, Raoul; Girgsdies, Frank; Prati, Laura; Schlögl, Robert; Trunschke, Annette (2013). "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol". ACS Catalysis. 3 (6): 1103. doi:10.1021/cs400010q. hdl:11858/00-001M-0000-000E-FA39-1.
- Csepei, Lénárd-István (2011). Kinetic studies of propane oxidation on Mo and V based mixed oxide catalysts. Technische Universität Berlin. pp. 157–166. doi:10.14279/depositonce-2972.
- Naumann d'Alnoncourt, Raoul; Csepei, Lénárd-István; Hävecker, Michael; Girgsdies, Frank; Schuster, Manfred E; Schlögl, Robert; Trunschke, Annette (2014). "The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts" (PDF). Journal of Catalysis. 311: 369–385. doi:10.1016/j.jcat.2013.12.008. hdl:11858/00-001M-0000-0014-F434-5.
- Dr. Tony Case (24 August 2018). Scientist Interview: Dr. Tony Case (Parker Solar Probe). Retrieved 24 August 2018.
- Vilaplana, J.; Romaguera, C.; Grimalt, F.; Cornellana, F. (1990). "New trends in the use of metals in jewellery". Contact Dermatitis. 25 (3): 145–148. doi:10.1111/j.1600-0536.1991.tb01819.x. PMID 1782765. S2CID 30201028.
- Vilaplana, J.; Romaguera, C. (1998). "New developments in jewellery and dental materials". Contact Dermatitis. 39 (2): 55–57. doi:10.1111/j.1600-0536.1998.tb05832.x. PMID 9746182. S2CID 34271011.
- Haley, Thomas J.; Komesu, N.; Raymond, K. (1962). "Pharmacology and toxicology of niobium chloride". Toxicology and Applied Pharmacology. 4 (3): 385–392. doi:10.1016/0041-008X(62)90048-0. PMID 13903824.
- Downs, William L.; Scott, James K.; Yuile, Charles L.; Caruso, Frank S.; et al. (1965). "The Toxicity of Niobium Salts". American Industrial Hygiene Association Journal. 26 (4): 337–346. doi:10.1080/00028896509342740. PMID 5854670.
- Schroeder, Henry A.; Mitchener, Marian; Nason, Alexis P. (1970). "Zirconium, Niobium, Antimony, Vanadium and Lead in Rats: Life term studies" (PDF). Journal of Nutrition. 100 (1): 59–68. doi:10.1093/jn/100.1.59. PMID 5412131.
External links
| Wikimedia Commons has media related to Niobium. |
| Look up niobium in Wiktionary, the free dictionary. |
- Los Alamos National Laboratory – Niobium
- Tantalum-Niobium International Study Center
- Niobium for particle accelerators eg ILC. 2005
- . Encyclopædia Britannica (11th ed.). 1911.
- Gilman, D. C.; Peck, H. T.; Colby, F. M., eds. (1905). . New International Encyclopedia (1st ed.). New York: Dodd, Mead.
- Niobium at The Periodic Table of Videos (University of Nottingham)
